Astiyak Ahmed, 14, has a monthly routine he can’t afford to miss. Since the age of two, he, along with his father, has been visiting the paediatric thalassaemia unit of Lokmanya Tilak Municipal General Hospital at Sion in Mumbai. Astiyak has thalassaemia major, a genetic disease that reduces the oxygen-carrying capacity of red blood cells, causing severe anaemia. Every month on the 21st or so, he undergoes blood transfusion. He spends those four hours playing games or watching movies on his father’s phone.
One could mistake Astiyak for an eight-year-old, owing to his frail figure—he weighs just 30kg—and short stature. His growth has been stunted, a common side-effect of the disease. “Most thalassaemia patients have iron depositions in their organs like liver and heart because of frequent blood transfusions. The iron deposition in the body also affects their thyroid and pituitary glands, affecting growth and weakening bones,” says Dr Mamta Manglani, head of paediatric haematology-oncology at the hospital. Though the excess iron is toxic, most patients have to undergo transfusions all their life.
Bone marrow transplants through siblings or a matching unrelated donor are the main cure for thalassaemia patients. Four young patients have been cured since last August thanks to the new bone marrow transplant unit at the Sion hospital. Patients have a 25 per cent chance of finding a human leukocyte antigen (HLA)-matched donor in their siblings. HLA are proteins or markers that help the immune system identify which cells belong to the body. Astiyak can't get a bone marrow from his sister as he is over the ideal age of eight and has hepatitis C, acquired from blood transfusion. For now, all doctors can do is give him blood transfusions and prevent complications.
A NEW TOOL ON THE BLOCK
Thalassaemia is caused by the mutation of a single nucleotide or letter, which is one of the building blocks of deoxyribonucleic acid (DNA contains our unique genetic code) and ribonucleic acid (RNA is like a copy of the DNA that carries instructions from it to make proteins), in the haemoglobin gene in a genome of more than 3 billion such letters. If doctors can somehow find a way to fix this mutation, the disease can be cured. CRISPR-Cas9, a new genome engineering tool, offers hope. If it becomes a part of clinical practice, patients like Astiyak would no longer need a bone marrow transplant. It would be possible to use the patient’s own cells to repair gene mutations.
 Mapping Disease: Prof S. Ramaswamy of the Institute for Stem Cell Biology and Regenerative Medicine | Bhanu Prakash Chandra
Mapping Disease: Prof S. Ramaswamy of the Institute for Stem Cell Biology and Regenerative Medicine | Bhanu Prakash Chandra
Such possibilities have, therefore, caused excitement in the scientific community. Hundreds of papers have been published since the tool was first developed in 2012. There is, however, a patent war waging between two renowned universities in the United States. Molecular biologist Jennifer Doudna of the University of California and Emanuelle Charpentier, now at Umea University in Sweden, published a paper in 2012 on the enzyme Cas9 that perfectly cuts the DNA. In 2013, Feng Zhang of the Broad Institute at Massachusetts Institute of Technology published a paper showing how CRISPR-Cas9 can be used on mammalian cells.
But the battle over ownership hasn't deterred numerous labs the world over to use the tool in their studies to uncover the mysteries lying in the genomic code of organisms. Among a flurry of research papers and ethical debates, some researchers claim the tool is ready to be used on humans. This July, Chinese researchers from Sichuan University were the first ones in the world to get approval to use CRISPR-Cas9 on lung cancer patients.
While most researchers think it is too early to be used in humans, CRISPR-Cas9 has immense potential. What makes it different from earlier genetic engineering tools like zinc finger nucleases and transcription activator-like effector nucleases (TALEN) is the ease of use and precision. What usually took specialised infrastructure with bulky machines and months of engineering in the lab can now be done in a matter of weeks in any well-equipped microbiology lab. It has brought down the cost and time significantly and has democratised gene editing. In fact, labs can even outsource the engineering part to companies specialising in it for a cost. It has become the most widely used tool for gene editing and has been announced as Science magazine’s breakthrough tool for 2015. There are speculations that the researchers behind the technique will soon win a Nobel Prize for their work, which would be a rare feat as it has been only four years since its discovery.
Already, CRISPR-Cas9 has been used to create genetically modified mosquitoes that do not spread malaria, repair genes that cause blindness, prevent HIV infections in cells, cure hepatitis and arrest the spread of cancer cells. It has been widely used in fruit flies, zebra fish and even primates. There was a furore when Chinese researchers announced that they have used the technology in human embryonic cells. Eyeing the innumerable commercial uses and popularity of this technique, investments worth billions of dollars ride on its research by pharmaceutical companies.
 Memory charm: Aparna Ashok of the National Centre for Biological Sciences, Bengaluru, is using CRISPR-Cas9 to better understand Alzheimer's disease | Bhanu Prakash Chandra
Memory charm: Aparna Ashok of the National Centre for Biological Sciences, Bengaluru, is using CRISPR-Cas9 to better understand Alzheimer's disease | Bhanu Prakash Chandra
WHAT DOES IT DO?
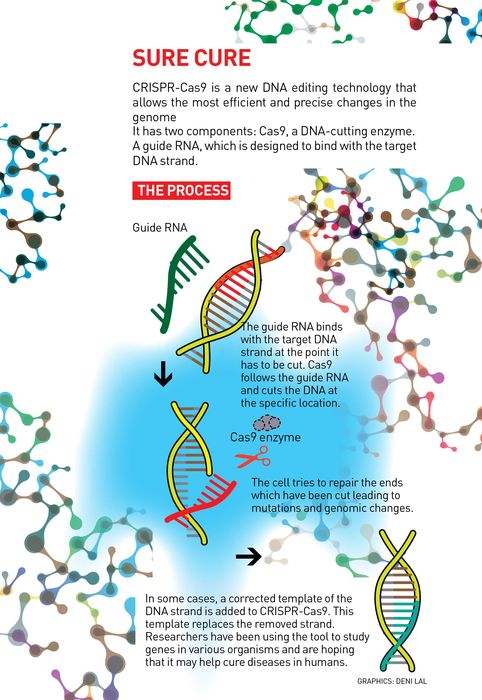
CRISPR-Cas9 was developed while studying the defence mechanism of bacteria against viruses. Scientists found that certain sections of DNA were frequently repeated in some bacteria. They called it ‘clustered regularly interspaced short palindromic repeats’ aka CRISPR. They later found that the bacteria use CRISPR as a database for the viruses it has encountered. When attacked by a virus, the bacteria use CRISPR to produce an RNA matching that of the virus. These together produce an enzyme Cas9, which cuts the genetic material of the virus, disabling it. If it can work in virus, it could also be used to cut any DNA at specific locations, including those of humans, thought researchers.
CRISPR-Cas9 has two components—a guide RNA that finds and binds to the desired location in the gene and Cas9 that cuts the DNA at the location. When the cell tries to repair at the cut, there are errors in the sequence that cause mutations, which disable the gene. This helps to study the impact of the particular gene on the organism. Alternatively, by providing a corrected template of DNA that fits at the cut ends, researchers are hoping to cure genetic diseases in humans.
ARE WE CLOSE TO A CURE YET?
Most of the work with the tool is currently being conducted on animals and in vitro in labs. Researchers say even though we sequenced the human genome 15 years ago, we still don’t know everything about human genes.
“Engineering comes from this understanding that makes you predict what kind of properties you will see. This kind of understanding does not exist in biology today,” says Professor S. Ramaswamy of the Institute for Stem Cell Biology and Regenerative Medicine at Bengaluru's National Centre for Biological Sciences (NCBS). “Just because we have the tools to build a bridge does not mean we should build a bridge when we don’t know how much load the bridge can take.” While he himself doesn’t work with the technology, he chairs the department of biotechnology’s task force on genome engineering. He says though the technology is still evolving and is not ready to be used in humans, it is still important as it helps ask specific questions about various life processes in organisms. With CRISPR-Cas9, it is now possible to tweak a single gene and study its properties or create conditions mimicking human disease in animals or stem cells. “Whether it is in plants or humans or animals, the understanding of mechanism by which a disease is caused is where the big impact is,” he says. “Only when we understand, can we fix the problem. You cannot correct in predictable fashion if you don’t understand. In the next 15 years, there will be a dramatic opening of our understanding of molecular basis of disease mechanism.”
WORK IN INDIA
A major breakthrough in stem cell technology along with CRISPR-Cas9 is responsible for this fast-developing field in genomics. In 2006, Japanese Nobel laureate Shinya Yamanaka and colleagues developed iPSC (induced pluripotent stem cell) technology, which showed that by increased expression of just four genes any adult cell can be converted into stem cells, capable of developing into any kind of cell. Researchers can, for example, take your blood cells and turn them into cells of the heart, liver or brain, which are genetically identical to you.
Using CRISPR-Cas9 and iPSCs, a team of researchers at NCBS and National Institute of Mental Health and Neurosciences are trying to better understand Alzheimer’s disease. APOE is a gene that is associated with the late onset of Alzheimer’s disease. APOE4 is a fairly common variant of this gene, present in 11-16 per cent of the population. Though having the variant increases the risk for the disease, not all those who have the variant end up with Alzheimer’s. Researchers—PhD scholar Aparna Ashok and Dr Mitradas Panicker of NCBS, Dr Sanjeev Jain, Dr Mathew Varghese and Dr Meera Purushottam of NIMHANS, and Dr Odity Mukherjee of InStem—are using patient samples to create stem cell lines with variants of APOE and study its impact on the disease. Ashok, who is the one using CRISPR-Cas9, says they have already edited the APOE gene within the stem cells since starting work about a year ago. This is the first Indian study using both genomics and biology to create cellular models of late onset Alzheimer’s disease, targeted at the Indian population, and is expected to provide important insights, says Mukherjee. This kind of specific targeted gene editing is achieved using CRISPR-Cas9. “Previously, you could do it [genome editing] but it was a herculean task,” says Panicker. “You had to use large numbers of cells, use complicated DNA constructs and the desired change occurred usually at very low frequency. With this technology, you can do it in a few weeks, be very precise and reproduce it very rapidly to confirm the results.”
This kind of precision is also helping Dr Deepa Subramanyam and her colleagues at the National Centre for Cell Science, Pune, ask vital questions about stem cells. They are trying to find out what kind of changes happen in the stem cells when they change from stem cells to other types of cells. They are specifically asking what role does the movement of molecules in stem cells play in their differentiation to other types of cells. Though they are studying stem cells of mice currently, the process would be similar for humans and plays an important role in making regenerative medicine safer, she says. Using fluorescent tagging of proteins, it is now possible to observe the changes in protein expression without fixing the cell that results in killing it. “We are using CRISPR-Cas9 to engineer specific mutations in genes that are of importance to us and engineer labels, so that you can track things in real time,” says Subramanyam.
Calling CRISPR-Cas9 a quantum leap for life sciences, Dr Sanjeev Galande, professor of biology at Indian Institute of Scientific Education and Research, Pune, says it has inspired a wave of studies on specific genes by altering them. His lab has used the technique to study how the embryonic stem cells change into all the other cell types and how cancer cells turn metastatic and spread to other tissues. His lab is also trying to understand the mechanism by which one X chromosome in females is turned inactive. There are about 12 projects using CRISPR-Cas9 in his lab of 22 researchers and they do the whole process in-house. “Once you adopt it for one process, it is easy. What changes is just the guide RNA. The technology spreads like wildfire in the lab. Once someone learns it in the lab, it is cool, others jump to learn it.” How popular a technology gets at the clinic level depends on how popular it is at the lab level, he says, “Now almost all modern labs have started thinking about this technology. It is just a matter of a few years by when it will be in the clinics or at least in clinical trials.”
CHALLENGES AHEAD
One of the crucial reasons scientists and researchers still aren’t keen to use CRISPR-Cas9 to edit genes in humans is because of ‘off-target effects’; despite its precision, the tool impacts other locations than the one intended, causing undesired mutations. Right now, researchers sequence the genome after editing to know whether it has been changed, but the full impact of the mutation in the gene may be understood only years later.
Dr D. Sundar, associate professor, department of biochemical engineering and biotechnology, IIT Delhi, and his team are working on designing a tool that will predict the ideal target for CRISPR-Cas9 and improve its overall accuracy. “When you are talking about using it on humans, it has to be more precise than it is and our tool will help increase its efficiency,” he says. This kind of focus on off-targets is uncommon and the paper on their tool will be published soon, he informs.
 Tracing Thoughts: Dr Odity Mukherjee of InStem, who is also working on the Alzheimer's study | Bhanu Prakash Chandra
Tracing Thoughts: Dr Odity Mukherjee of InStem, who is also working on the Alzheimer's study | Bhanu Prakash Chandra
Another challenge is that editing the gene in all the billions of cells of the body is an uphill task. “So you have to start in a place where the cells can multiply and become larger and larger in numbers, like the bone marrow,” says Galande. “But still, there are issues like how you can deliver therapy there. This is a scientific issue that has to be worked out together by researchers in labs and the doctors in clinics.” Another widely cited concern by scientists is ‘gene drive’, where the edited genes are inherited faster than in nature, possibly dominating the gene pool.
WHERE DO WE STOP?
While it may take 10 to 15 years to see it being applied in humans, plenty of revolutionary research is being done with the technology across the world. MIT Technology Review had reported that three US institutes were working on growing human tissue inside pigs to harvest organs like liver, heart and kidneys for transplantation using CRISPR-Cas9 and iPSC technique. Another team from South Korea created 'double-muscled' pigs by genetic editing that would be suitable for human consumption. Some Chinese companies like BGI are using the gene editing technique to create tiny pigs to be sold as pets. A Harvard Medical School team claims to have created elephant cells with 14 genes from woolly mammoth that can increase cold tolerance. There are plans to release endangered Asiatic elephants tolerant to cold in north Siberia.
However, not everyone is excited about some of these eccentric propositions, with fiery ethical debates raging on how far we should attempt to meddle with our genes. Many fear that the gene editing tool might end up in wrong hands and lead to dangerous consequences. Others say there will be a demand to create tailor-made babies with desirable traits. A few others are scared about the impact of these organisms on the environment. Last December, a group of ethicists and researchers from the US, China and the UK debated on using the gene editing tool in human beings. They agreed that research could be done on human embryos and germ-line cells—sequence of cells that develop into eggs and sperms—but these embryos cannot lead to pregnancy.
Also, each country has its own guidelines in dealing with these tricky issues. The US National Institutes of Health do not fund any research involving the use of human embryos. It is, however, allowed in China. The Indian Council of Medical Research guidelines, on the other hand, prohibit germ-line cell editing.
Some scientists don’t think the fear and hype are justified. “Historically, every time there has been a breakthrough technology, the end-of-the-world prediction has been constant. But it has never happened,” says Ramaswamy. He says the possibility of someone creating something dangerous from the technology is infinitesimally small and should not be used as an excuse to unfairly restrict the progress of science.
While agreeing that it is too early for CRISPR-Cas9 to be used in humans, Subramanyam says we need to have faith in regulatory bodies to ensure that it is not used before the required approval. “Because the flip side is so much more powerful," she says. "If you can generate a type of tissue that will help a sick individual, I think that type of power is so much greater than the possibility that there will be some crazy person who would want to clone people.”
WHAT IS A GENE?
Each cell of our body contains a genome or a complete set of DNA in its nucleus. Each strand of DNA contains millions of tiny nucleotides named A (adenine), C (cytosine), T (thymine) and G (guanine). A gene is a part of the DNA that is coded for information that builds a specific protein using the four letters. Human beings have 20,000 genes and many of them are responsible for our health.
WHAT IS GENE MUTATION?
Gene mutation is a change in the DNA sequence of A,C,T, G owing to errors in DNA replication or owing to environmental factors. This results in a change in the kind of protein synthesised. Most gene mutations are detected and corrected by the cells, while a few become permanent. Gene mutations cause genetic variation in the species and can be inherited. Some mutations cause diseases.
WHAT IS GENE EDITING
It is a type of genetic engineering where parts of DNA are edited, inserted or removed using molecular scissors called nucleases. There are three types of genome-editing tools—zinc finger nucleases (ZFNs), transcription activator-like effector nucleases (TALEN) and the latest CRISPR-Cas9. CRISPR-Cas9 is the cheapest, fastest and the most precise of the tools. CRISPR-Cas9 can be used to cause changes at multiple locations at once, which help to study and understand complex genetic conditions.