Cancer, for Kusum, shed its cloak of invisibility with a facial twitch in 2012.
The year was supposed to be one of new beginnings for Kusum and her husband, Vivek Tomar. Married for four years, the couple had been living apart owing to jobs in different cities—Vivek, a pharmaceutical graduate, had been working in Hyderabad, and Kusum was a schoolteacher in Rohtak, living with her parents and son. Gurgaon brought them together; they both found jobs here. They were out shopping, looking for furniture for their new home, when Kusum's right cheek started twitching.
Kusum, 32, was initially diagnosed with tuberculosis of the brain. But her condition worsened after she started taking anti-TB medicines. Two months later, she was diagnosed with non-small cell lung cancer. “A radiologist saw a patch on Kusum’s lungs during a full body MRI scan. A rare form of cancer, it had already spread to her brain and formed multiple nodules there,” says Vivek.
Kusum underwent chemotherapy, but the drug reacted adversely after the third chemo cycle. It led to accumulation of fluid around her lungs and almost choked her to death. “I am one of those few people who show resistance to chemotherapy. So, the doctors prescribed me the next line of drug—crizotinib,” says Kusum. The drug kept the cancer under control for the next 22 months, but she developed resistance to it as well. There was no next-generation drug available in India, and without treatment, she was told she would live only for four more months. However, the drug she needed—ceritinib—was available in the United States. But importing it would have cost them 110 lakh a month, which was way beyond their means.
 Musical care: Meryl Mammen at her Ghaziabad home. She has Pompe disease, a rare disorder in which the body cannot make an enzyme required to break down the complex sugar glycogen | Arvind Jain
Musical care: Meryl Mammen at her Ghaziabad home. She has Pompe disease, a rare disorder in which the body cannot make an enzyme required to break down the complex sugar glycogen | Arvind Jain
Vivek met doctors and wrote letters to various pharmaceutical companies to know how ceritinib can be brought to India at a reduced price. They told him that a drug could be brought to India only after it cleared the clinical trial phase here. The pharmaceutical company manufacturing the drug was awaiting the approval of the Drug Controller General of India (DCGI) to conduct trials. Vivek wanted to accelerate the process and he wrote to the DCGI. Finally after four months, the clinical trials began and Kusum was put on the drug.
Kusum responded well to the drug. The tumours in her brain shrank. The cancer in the rest of the body was arrested and there was improvement in her condition. There were side-effects, of course—nausea, vomiting, drowsiness and epileptic convulsions. But she fought them all. A pink bicycle in the lawn stands testimony to her determination. When she started feeling weak, she asked Vivek to get her a bicycle so that she could build her stamina. “I want to live for my son and my husband. I can fight all odds to be with them,” she says.
Kusum's cancer, however, continues to be invincible; she has developed resistance to ceritinib as well. She now needs the next line of drugs—alectinib (available in the US market), brigatinib (cleared clinical trial) and lorlatinib (under clinical trial)—which are far from coming to India. “I can fight the side-effects and keep myself motivated, but how can I fight cancer without any drug?” asks Kusum. “There are so many advanced medicines in the US and so many more in the pipeline. The life-expectancy of someone with my kind of cancer is at least ten years. How can our government not think of bringing these lifesaving drugs to India when all other countries have them?”
 THE WEEK, in its cover story dated March 6, 2011, highlighted how the clinical trial industry had become a money-making business.
THE WEEK, in its cover story dated March 6, 2011, highlighted how the clinical trial industry had become a money-making business.
Kusum's story highlights the desperation of thousands of patients who live with diseases for which there are no medicines available in the country. It is ironical that the largest producer of drugs in the world finds it difficult to introduce new drugs in its own market. All because the process of getting permissions to conduct clinical trials—the only gateway through which a new drug can enter the Indian market after proving its efficacy and safety in the Indian population—is quite cumbersome.
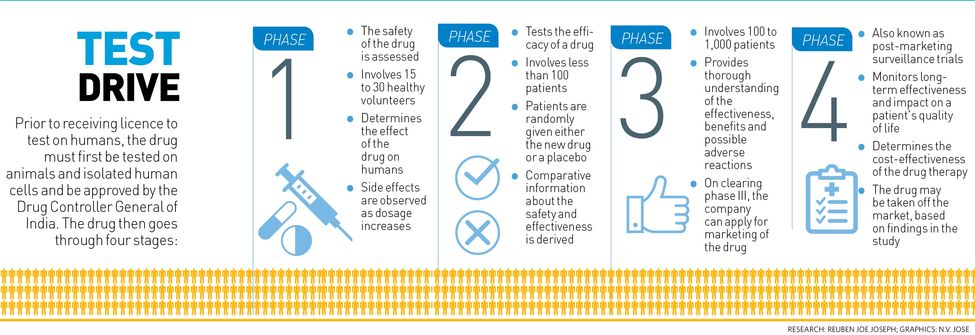
After a drug molecule is tested in animals and found safe, the pharmaceutical company sends an application to the DCGI for conducting phase-1 clinical trials. Once the approval comes in, the company chooses doctors as principal investigators, who are trained by the company on the protocols for the trial. The investigators give applications to the ethics committees, comprising groups of experts from various fields, of the clinical trial sites. The committee assesses the risks and benefits of the drug under study, and if found safe, grants permission for the four-phased trial. The investigator then enrols patients and starts the trial. The sponsor of the study (the pharma company, in most cases), the DCGI inspectors and members of the ethics committee keep a close watch throughout the trial.
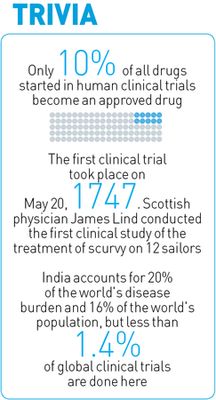
In India, clinical trials have lacked regulation for long. For nearly a decade till 2011, it was easy to obtain permission to conduct clinical trials. According to the DCGI, the government approved 475 clinical trials for “new chemical entities”, not used as a drug elsewhere in the world, between January 2005 and June 2012. There were reportedly 11,972 cases of adverse effects excluding deaths, 506 of these being directly attributed to the trials. Besides, 2,644 deaths were reported between 2008 and 2013. Among them, four tribal girls, aged between 10 and 14 years, from Andhra Pradesh and Gujarat who died allegedly during the trials of a Human Papillomavirus vaccine for cervical cancer.
THE WEEK, in its cover story dated March 6, 2011, highlighted how the clinical trial industry in India had become a money-making business, which had led to the exploitation of poor and vulnerable patients. There were no stringent laws to guarantee adequate compensation to the patients in case of an adverse event owing to the drug under trial.
In 2011, many NGOs protested the malpractices prevailing in the sector. A petition was filed in the Supreme Court, which blamed the government for the laxity in the norms. In early 2013, the court appointed a committee, headed by Professor Ranjit Roy Chaudhury, a noted pharmacologist, to come up with recommendations to improve the system of drug development and clinical trials in the country. As per its recommendations, the permission of the health secretary had to be sought, apart from the DCGI's approval for trials. However, objections were raised when the health ministry gave approval to 162 global clinical trials in less than two months the same year. Even as the government and court were charting out the new roadmap for clinical trials, many pharmaceuticals and research institutions like the US-based National Institutes of Health cancelled many of their trials in India.
Then came a set of new rules and regulations, which were anything but clinical. The regulations required registration of all ethical committees with the Central Drugs Standard Control Organization (CDSCO) of the Union health ministry, audio-visual recording of patients’ consent, involving insurance companies for providing compensation to patients in case of an adverse event and so on. “Everything came to a standstill for three years. It was a major setback for clinical research and new medicines in the country,” says Dr C.S. Pramesh, thoracic-onco surgeon at Tata Memorial Hospital, Mumbai.
Agrees Dr Vyankatesh Shivane, a diabetologist at KEM Hospital, Mumbai, who stopped conducting trials after the new regulations came in. “My private clinic was approved by the US Food and Drug Administration, but the new Indian rules are difficult to follow. I have conducted more than 45 trials, of which 12-13 drugs came to the market.”
Clinical trials are important to meet India's requirements for new drugs for diabetes, he says. “Unlike the US, where insulin is the preferred line of treatment, people here want to delay its use. Besides, the patho-aetiology is different. People with low body mass index also have high central fat,” says Shivane. “Cost is another major factor that pharma companies have to take into account before coming up with a new drug here.”
The most affected are cancer patients and people suffering from terminal illness, rare disorders, severe infections and drug resistance. The only way to deal with resistance to drugs and treat newer and rarer diseases is to build a parallel process of not only developing new drugs but making it available in the country. Take the case of Ghaziabad resident Shashank Tyagi, who has Gaucher's disease, a rare genetic disorder caused by the abnormal buildup of fatty substance in organs. “I was bedridden and totally dependent on others for my daily chores. There was no drug to help me. When the clinical trial for enzyme replacement therapy was held in India, I enrolled myself. I am independent now,” he says.
Meryl Mammen, however, had trouble finding a suitable trial for Pompe disease, a rare disorder in which the body cannot make an enzyme required to break down the complex sugar glycogen. Enzyme replacement therapy, the only treatment available, costs 12.6 crore per year. “I would not have survived without medicine,” says the 26-year-old Ghaziabad resident. “My father's company has been paying for my medicine for the last five years.”
Prasanna Shirol, founder of Organization for Rare Diseases in India, says there are more than 7,000 rare disorders reported in India. Of these, drugs are available for only 500. “In Gaucher’s disease, for example, if 300 children are diagnosed, only 80 per cent can get medicine either through clinical trial or government aid. The rest suffer before they succumb,” says Shirol, whose 16-year-old daughter, Nidhi, suffers from Pompe disease.
Drugs in case of a rare genetic disorder may not cure but arrest the symptoms and help in improving the quality of life. In a standard process, it takes seven to ten years for a molecule to become drug and be available in the market after clearing clinical trials. People like Srinivas Bitla of Mumbai are well aware of the consequences of such a long drawn-out process. Bitla’s son Nihal lost his life to Hutchinson Gilford Progeria at 13. In the rare disorder, a child ages rapidly. There was no treatment available until a year ago, when he was called by Boston Children Hospital and Progeria Research Foundation to participate in a clinical trial in Boston for a new drug called Lonafarnib. The medicine worked wonderfully on him. Nihal got flexibility to bend forward and his negligible teeth line got its structure and colour back.
In March, however, Nihal died of dehydration. “He didn’t die of progeria,” says Bitla. “The medicine under clinical trial improved his quality of life tremendously. I have decided that I will spread awareness about the importance of clinical trials so that parents of other children suffering from progeria do not say no to available medicine.”
 Support system: Prasanna Shirol with his daughter, Nidhi, who suffers from Pompe disease. Shirol is founder of Organization for Rare Diseases in India | Jagadeesh N.V.
Support system: Prasanna Shirol with his daughter, Nidhi, who suffers from Pompe disease. Shirol is founder of Organization for Rare Diseases in India | Jagadeesh N.V.
From rare disorders to the most common infections, each disease is seeing a progression that is making it challenging for the medical fraternity to treat it.
Another important aspect of getting newer medicine is to reduce the cost of the treatment of chronic diseases. Dr Sneha Limaye, head of clinical trials at Chest Research Foundation in Pune, says 15 of 40 new molecules her institute conducted trials on in the last ten years have become the drug of choice in many cases. It is only because of research, says Limaye, that the cost of the treatment of chronic obstructive pulmonary disease, the second largest killer disease in India, has come down to 13 per dose.
“A doctor feels good when the molecule he or she has tested changes the life of the patients,” says Pramesh. “Participating in a clinical trial is an additional responsibility for a doctor. Most doctors do it only when they are passionate about research. But it is necessary to have a population specific data for a drug to document its efficacy and safety in a particular population.”
He cites the example of the global trial of Gefitinib, a drug for lung cancer, in which the Tata hospital had participated. “The drug was found to be ineffective in most countries. But while documenting the data, it was found that the drug was quite effective in a sub-group consisting of non-smoker Asian women. Though dismissed by the whole world, Gefitinib became the drug of choice for us in India,” he says.
In a survey conducted by Chest Research Foundation last year, most doctors who work as principal investigators in a clinical trial agreed on the central registration of ethics committees and on improving the mandatory compensation to the subjects for study-related serious adverse events. A majority, however, did not agree to making it compulsory to include government sites in clinical trials and the introduction of audio-video recording of informed consent. “Though the move to record the consent of the patient is to safeguard the patient’s right, it makes the whole process cumbersome. Patients get worked up and get suspicious about the safety of the trial,” says Limaye. “Besides, they generally have many personal queries regarding the side-effects of drugs on their sex life and child-bearing, which they are not comfortable asking about on camera and become reluctant in participating.”
The process of enrolling people in a clinical trial is different in India and the developed countries. In the US, the information about any ongoing or upcoming clinical trial is simply put on the institute’s website and around the campus. Interested patients themselves contact the doctor, who then explains the intricacies of the clinical trial. This way, patients have much more trust on the doctor. In India, it is the doctor who has to approach each and every patient.
The new guidelines unfortunately have also halted academic research in India. The Tata hospital, in collaboration with Medical Research Council, UK, was to begin research on how Aspirin, a popular blood-thinner, can be used as an anti-cancer drug. “There is a large data to support the initial research. It took both the institutes two years to draw all the protocols. Though the research has already begun in the UK, we are still waiting for the approval,” says Pramesh. “Can you imagine the impact this kind of a study will have in bringing down the cost of cancer treatment?” There is no comparison between cancer research in India and across the world, he says.
Even hardcore research institutes are finding it difficult to adhere to the government’s guidelines. Dr Raman Gangakhedkar, director, National AIDS Research Institute, Pune, says they have conducted eight to nine clinical trials in the last ten years. But of late, it has become difficult for them to participate in any clinical trial because of the strict compensation law. Relaxation of these rules is given in four conditions—epidemic, national emergency, extreme emergency and rare disorders. Now, HIV doesn’t fall in any of these categories, and even insurance companies don’t provide insurance to people with HIV, he points out. “How can we adhere to this rule? Should research in this vital medical field halt for such a reason?” asks Gangakhedkar. “The west prefers medicines that are less toxic and more effective. Unfortunately we still have to look at the cost of the medicine and what is available in India.”
 Cure call: Srinivas Bitla with his son Nihal, who took part in a clinical trial for a new drug for progeria in Boston. The drug worked, but Nihal died of dehydration in March.
Cure call: Srinivas Bitla with his son Nihal, who took part in a clinical trial for a new drug for progeria in Boston. The drug worked, but Nihal died of dehydration in March.
Dr Soumya Swaminathan, director general, Indian Council of Medical Research ( ICMR), says she has been working towards bringing down the approval time for conducting a clinical trial to six months. Also, she says it is important to assess whether deaths and other adverse events related to clinical trials in the past occurred due to the drug under study or because of any other factor. “Take, for example, the HPV vaccine against cervical cancer. There were deaths reported in tribal girls in Andhra Pradesh. But it is important to take into account the death rate under the normal circumstances among the population under study,” she says.
Though it is important to make stronger rules to protect the rights of participants, Swaminathan says drug trial is equally crucial to protect people against deadly diseases. “We couldn't participate in the global trials for multi-drug resistant tuberculosis of which South Africa was a part. Don’t we need newer drugs to address the growing problem of extreme drug resistant tuberculosis?” she asks.
Dr G.N. Singh, drug controller general of India, however, says the period of uncertainty and delay will be over shortly. The regulatory body is in the process of making the system of obtaining permission for clinical trials robust, fast and transparent. “Indeed, India has a unique disease burden and it needs to be addressed with country-specific drug trials. I think in the next two-three years, we should be back on track as far as clinical trials and bringing newer drugs to the Indian market is concerned.”
In early August, the CDSCO issued two circulars, giving the ethics committee the power to decide on the number of trials a principal investigator can undertake and also to choose the site of the trial. Earlier, an investigator could not work on more than three trials at a time, and only hospitals with minimum 500 beds were allowed to conduct trials.
Though these are steps in the right direction, it is high time India got clinical about drug trials.







