The World Health Organization (WHO) has recommended widespread use of RTS,S/AS01 malaria vaccine, which is also known by its trade name Mosquirix.
WHO Director-General Tedros Adhanom Ghebreyesus called it “a historic moment” after a meeting in which two of the UN health agency’s expert advisory groups recommended the step.
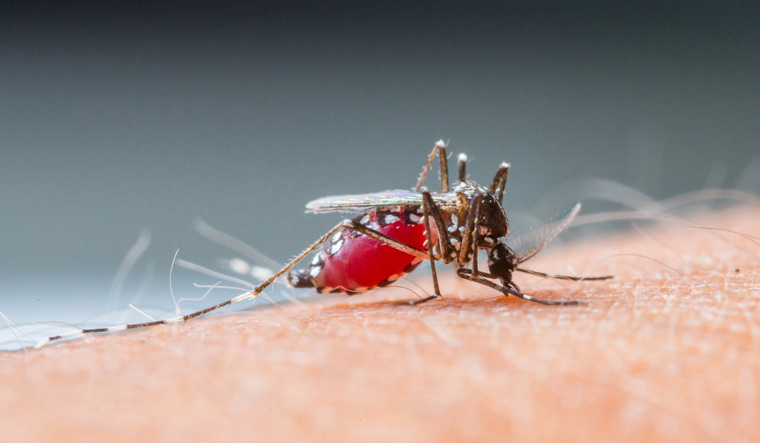
Malaria is caused by a parasite known as Plasmodium, which is transmitted to human by the bite of the female Anopheles mosquito. Plasmodium enters the mosquito when it takes a blood drink from an infected human, mammals, reptiles and birds.
Designing a shot against malaria was particularly difficult because it is a parasitic disease spread by mosquitoes and not caused by a virus or bacteria.
Mosquirix is the only vaccine that has demonstrated it can significantly reduce malaria in children. In clinical studies, it was found protect 40 percent children who are immunised and has reduced cases of life-threatening severe malaria by nearly a third.
scientists say the vaccine could have a major impact against malaria in Africa, home to most of the world’s more than 200 million cases and 400,000 deaths per year.
Azra Ghani, chair of infectious diseases at Imperial College London, said she and colleagues estimate that giving the malaria vaccine to children in Africa might result in a 30 percent reduction overall, with up to 8 million fewer cases and as many as 40,000 fewer deaths per year.
RTS,S/AS01 malaria vaccine has been developed after two decades of studies by GlaxoSmithKline (GSK) and the Walter Reed Army Institute of Research (WRAIR) in collaboration with the Bill & Melinda Gates Foundation at a cost of US$700 million.
The scientific name RTS,S derives from its composition. RTS,S is based on the major Plasmodium falciparum sporozoite surface antigen, circumsporozoite protein (CSP). Here, 'R' stands for the central repeat region of Plasmodium (P.) falciparum circumsporozoite protein (CSP); the 'T' for the T-cell epitopes of the CSP; and the 'S' for hepatitis B surface antigen (HBsAg).
Immunity to a pathogen can be acquired by natural exposure to the microorganism or through vaccination.
RTS,S aims to trigger the immune system to defend against the first stages when the Plasmodium falciparum malaria parasite enters the human host's bloodstream through a mosquito bite and infects liver cells.
RTS,S malaria vaccine has proved to enhance the production of protective antibodies upon subsequent parasite infection. By preventing or significantly decreasing the load of parasites emerging from the liver, vaccination with RTS,S reduces the rates and severity of clinical malaria disease.
By inducing the immunity, the vaccine breaks the cycle of malaria parasite between humans and mosquitoes. Thus the vaccines is a tool to reduce the mosquito infection and malarian transmission.
“This is a huge step forward,” said Julian Rayner, director of the Cambridge Institute for Medical Research, who was not part of the WHO decision. “It’s an imperfect vaccine, but it will still stop hundreds of thousands of children from dying.”