There was a switch from the regular toothbrush to an electric one, perhaps because a pinched nerve made the up-down, side-to-side movement of the regular toothbrush harder than usual. Then there was a tingling sensation in the right leg, and soon in the left leg as well. So, the obvious thing was done—the one that doctors hate—check for symptoms on the internet. Amyotrophic Lateral Sclerosis (ALS), it said. Petrified, a visit to a neurologist was made. The right way of diagnosis was suggested—a scan. The MRI showed that it was Parkinson’s disease, and not ALS. From the initial denial to acceptance in the next couple of months and then figuring out a way to live with it, the journey is nothing less than difficult. After all, they say, Parkinson’s is not a death sentence, but a life sentence—you do not die of the disease, you die with it.
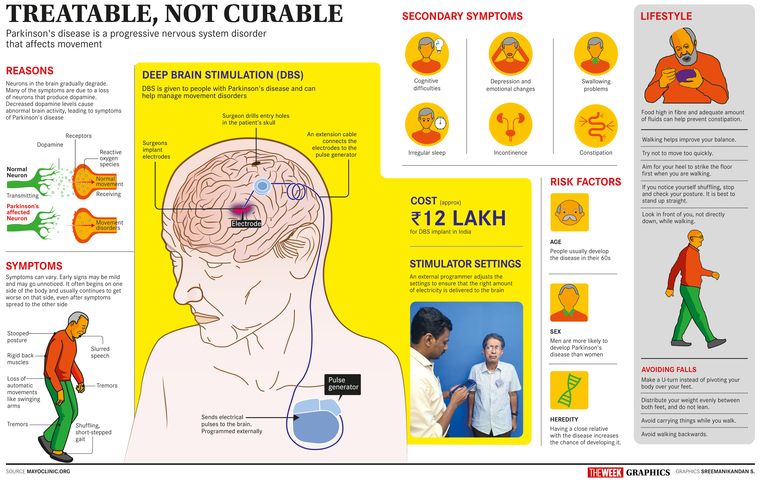
In 1817, when British apothecary James Parkinson published his ‘An essay on the shaking palsy’, where he gave a detailed description of the condition, little did he know that 48 years later the disease would bear his name. Today, an estimated one crore people worldwide are living with Parkinson’s disease. It is a progressive nervous system disorder that affects movement. From barely noticeable symptoms like slight tremors in one hand to regular tremors, stiffness and slowing of movement, the symptoms worsen as the condition progresses over time. Although Parkinson’s cannot be cured, medication might improve symptoms. And when the abnormal movements cannot be managed by medicines, the treatment is taken on-site.
At the Sree Chitra Tirunal Institute for Medical Sciences and Technology (SCTIMST), Thiruvananthapuram, director Dr Asha Kishore, also a movement disorder specialist, and her team of engineers and doctors are working with engineers from the Bhabha Atomic Research Centre (BARC) on the first indigenous advanced technology in the field of neuroprosthetics—a new and improved Deep Brain Stimulation (DBS) system for movement disorders such as Parkinson’s disease, essential tremor and dystonia. “DBS treatment has been around for the past 20 years, both in the west and in a few institutions in India,” says Kishore. “In the Comprehensive Care Centre for Movement Disorders (which she heads), about 300 patients have benefited from the technology. The SCTIMST was the first in the country to provide this treatment for Parkinson’s disease.”
According to Kishore, the teams at BARC and SCTIMST have started with optimisation of existing technologies to make it simple, user-friendly and affordable. “In India, the cost of rechargeable DBS with a 15-year life span is about Rs12 lakh, but if it can be manufactured and sold at even half the price, it will cater to a larger segment of patients,” she says. The two teams have already put in three years of work into the project, and are quite optimistic about its success. “Right now, the prototype is getting ready and we are looking at 2022 for clinical testing of the device. But I am not at liberty to talk a lot about it as we have an agreement with our partners and it is a little too early,” says Kishore.
In DBS, a set of platinum electrodes on a thin, insulated wire are implanted in both halves of the patient’s brain. The electrodes are inserted through small holes made at the top of the skull. An MRI is used to identify the site for the electrodes, which are connected through a cable that travels under the skin of the neck to a battery-powered pulse generator implanted under the skin of the chest. “The device operates on a lithium-powered battery, which is not manufactured in India and had to be imported,” says Kishore. “When it comes to placing the pulse generator under the skin, extra care needs to be taken so as to avoid fluid from entering the battery. In order to avoid this, a new sealing technique has been used in our prototype. It is a very high-risk device and we are looking at absolute safety when it comes to the electrodes, pulse generator, wireless recharging and the associated electronics.”
In all DBS systems, low-energy electrical stimulation is given to the brain using the pulse generator. These electrical impulses modify brain activity in a controlled manner, by blocking faulty nerve signals that cause movement disorders like tremors, stiffness and walking problems. “After the activation of the stimulator, the doctor programmes it from outside the patient’s body using a controller,” says Kishore. “We regulate the amount of stimulation according to the requirement for each patient.” According to her, DBS treatment offers significant relief from symptoms that stop patients from living a normal life. DBS can also reduce dependence on drugs. Patients can enjoy good quality of life for 10 to 15 years, if it is done before the disease is too advanced. In later stages, when it has spread to other parts of the brain, DBS alone may not be enough. DBS can now also be used to treat obsessive compulsive disorder. “Researchers are also looking at the effects of DBS for intractable epilepsy, depression, bipolar diseases and even drug addiction,” says Kishore. Currently there is limited application, but Kishore says that DBS is going to be a technology that will have widespread application in the future. “Right now, we look at the patient’s symptoms and set the stimulator, but the new model of DBS under development will sense what is happening in the brain when symptoms appear and provide stimulation for the time that it is required and not continuously, thus saving battery life,” she says.
While high-risk device development is not new to SCTIMST, Kishore says that they have the best research partners in BARC. “[But] it is not an easy task to find a suitable medical device industry partner to transfer the technology to manufacture the device and commercialise it,” says Kishore. “We have a technology business division for that.” The research teams work closely with the industrial unit to train them in manufacturing the product.
The SCTIMST was declared an Institute of National Importance in 1980 under the Union government’s department of science and technology (DST). According to Kishore, the institute has a unique mandate that combines advanced patient care in medical specialities and research in medical sciences, biomedical technology development and health sciences under a single institutional framework. “The Rs100 crore funding under the Technical Research Centre for Biomedical Devices by the DST has been a big boon for us,” she says. “It has kickstarted some of the breakthrough projects in our institute. We want our team of researchers to think beyond the norm and innovate radical technologies at affordable prices.”
From disposable blood bags, the first product developed by SCTIMST to the TTK-Chitra heart valve, the institute has had some successful commercialisations in the past. These products have fared well in the market, competing with well-established and imported brands. “It has been 28 years since the launch of the TTK-Chitra heart valve and more than 1.5 lakh people around the world have benefited from it,” says Kishore.
Currently, SCTIMST is concentrating on regenerative technologies and spinal fixation devices and orthopaedic implants to address geriatric needs. “In 2020, we plan to start work on artificial joints, bone fixation devices, spine correction and exoskeleton electromechanical devices that can help people who are paralysed from the hip down to stand on their feet,” says Kishore. The SCTIMST has already transferred 18 devices or biomaterials in the last three years and plans on transferring at least 34 new technologies within the next five to seven years. Of these, 16 are procedure-based projects. “High-risk technologies are our forte. But next year, we are planning on transferring 15 technologies that are low risk and low-moderate risk,” she says. “We are excited about the number of patents that some of our researchers have filed and are going to file. It is great to know that scientists are now sensitised about the right way of documenting its work.” In a bid to achieve their technology transfer target, SCTIMST is gearing up on all fronts such as infrastructure and facilities, human resources, external expertise and networking with higher centres of learning and excellence.
With the number of lifestyle disorders and degenerative diseases of the elderly set to increase in the coming years, Kishore says that they have a big responsibility to provide affordable medical devices that are crucial in prevention, early detection, treatment and rehabilitation. “Now, most of the medical devices are imported from the west,” she says. “We are working towards a future in which our country is self-sufficient to meet its medical devices needs. Discover, design and develop—this must be the chant of every innovator and researcher in this field in the country,”
Looks like the next couple of years are going to be revelatory for the medical field in India. Keep an eye out for the ‘Made in India’ tag!