You wanted to release ZyCoV-D on Gandhi Jayanti. Was it delayed because of pricing issues?
Our original plan was to release it in October. Now we are waiting for the pricing approval. It is more complicated because there are three doses plus the injector plus the adapters. We hope to clear all that by this week and launch it as soon as it is done. It will be competitively priced. Our pricing is based on the technology, scale, capacity and procurement. There are a lot of advantages to this vaccine, and not only for today. It is a platform for the future.
How many doses/batches are you introducing for the drive this month?
Currently we are in the scale-up process with our new plant that got delayed so our commissioning of the new facility which happened in the April-May-June period got delayed by a month because there was a heavy outbreak of Covid and so we had that impact too. So now in October we are scaling up and I'm hopeful that we achieve 2 to 3 crore doses by the end of December this year and that's what we are targeting.
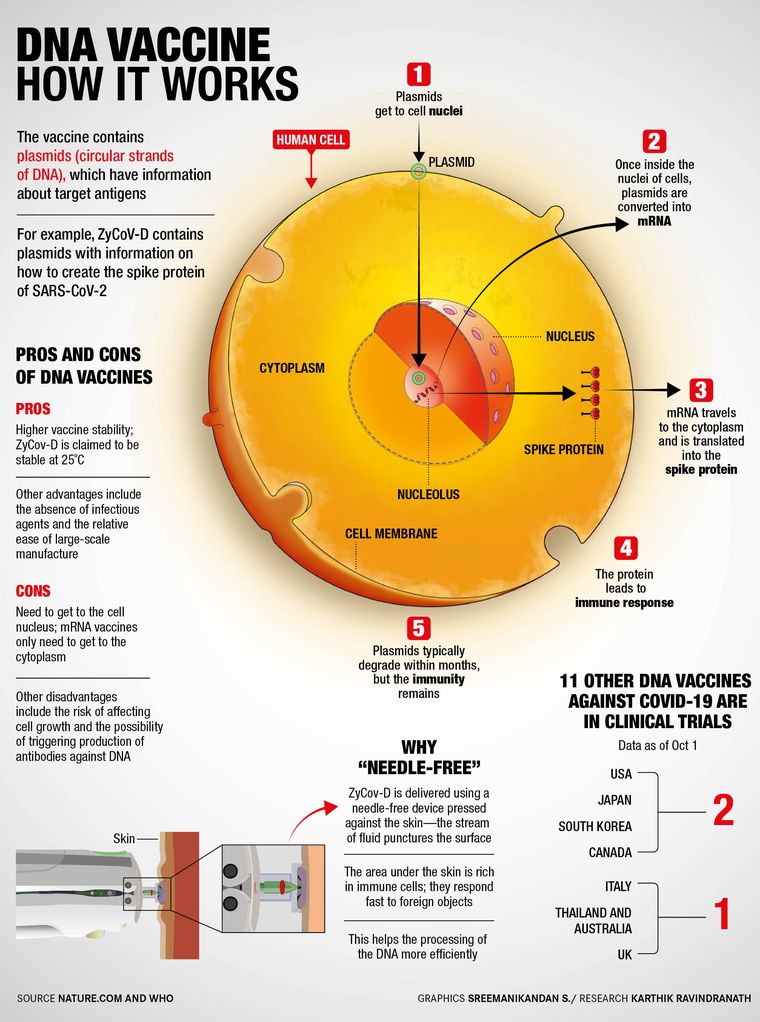
Tell us about plasmid DNA, the technology behind world's first such vaccine.
This vaccine is focused on the virus’s spike protein. When administered in a human body, the ZyCoV-D will produce the spike protein of the SARS-CoV-2 virus. There has been great interest in this technology because of its ability to elicit both humoral immune responses (relating to immune responses involving antibodies in body fluids) and cellular immune responses (antibodies in cells) and in showing relevant advantages regarding producibility, stability and storage.
The good part about this is that the vaccine constructs, which are generated quickly to deal with mutations in the virus, are non-replicating and there is no vector-based immunity built because we don’t use anything other than the spike protein. So there are no adjuvants (an ingredient used to create a stronger immune response) and no infectious vectors. All of it makes the administration of the vaccine easier. [The side effects, too, have been the lowest].
This is also a thermostable vaccine, which is stable at 2 to 8° Celsius (refrigeration temperature) and even at 25° Celsius for three months. Open vial studies have shown that they are stable for at least a week, so you don’t have to give the dose to everyone the same day [after opening a vial].
Then, of course, the vaccine is intra-dermal, which means a needle-free device is used for the application, minimising local side effects. When we inoculated 28,000 volunteers including 1,400 children, we did not have a single dropout. It was very well tolerated by all volunteers. Also, the great advantage of the DNA strategy is the short time required from design to clinical trials. Therefore, it may soon be possible to test together, in the same vaccine, different variants of antigens that cover circulating mutations.
There were no side effects at all?
No, side effects could be there but the side effects between the placebo and the vaccine are similar or less and the overall assays are in single digits with very significantly low side effects.
Several companies across the world use the DNA plasmid technology, but you have outdone them.
This technology has been around since the time of the Ebola outbreak in the early 2000s. That was also the time when the World Health Organization said DNA technology could be a good platform to deal with viruses of such nature. The WHO has revised its guidelines twice on DNA platforms and has cleared all DNA vaccines for their safety. Even the US FDA has published its update on the DNA plasmid vaccine and considers this platform to be very safe.
Also read
- Canada to discard 13.6 million Covid vaccine doses
- COVID-19: Why the next three months are crucial
- Virus continues to be highly transmissible: ICMR-NIV chief Dr Priya Abraham
- What it means to work in a Biosafety Level-4 laboratory at NIV
- Kerala will return to normalcy by November: Health Minister Veena George
- Fewer cases mean fewer mutations, says AIIMS director Dr Randeep Guleria
At present, 14 trials are being undertaken across the world by different companies of which some are in phase 3. So it is not that this is unexplored, but we were able to do it in a shorter period of time. We were the first company in India to do the H1N1 vaccine and that was also done in a record period of nine months. Our R&D and execution efforts have been good and that is why we have done things earlier and faster.
Has this been a long time in the making? Also, how did you decide in favour of the DNA tech?
We started the development of this vaccine in February 2020, but the technology for DNA development has been around for the last 12 years. Of course, it was a big risk to pick up a platform for which currently there are no approved vaccines, but there were a lot of benefits in picking it up. When we looked at the virus which came from a family of coronaviruses, [we saw] all of them being significantly prone to mutations. So we felt we needed a platform that could be easily interchangeable to the developing viruses as they mutate. So all you needed was to change the sequence of the strains and your whole model, construct and process remain the same. So it was a plug-and-play technology wherein all you have to do is to change the gene of interest by the mutation that happens. You are basically not changing anything that is part of the vaccine platform. So, that was one important consideration.
The other consideration was that if it became a critical vaccine, then its supply chain would become critical and we knew that this is one of the vaccines that is thermostable. Finally, as a company we have always believed in innovation and bringing to the fore newer technologies and newer drug discoveries. We wanted to demonstrate and build a capability for newer technologies and that is why we picked up the DNA one versus the RNA one. The RNA-based one was also very interesting, but we knew about the challenges regarding cost and thermostability. We were also contemplating the measles platform. So we picked up two platforms to work with, but our earliest animal studies showed that the plasmid DNA model was far superior.
How convincing were the pre-clinical or animal study trials?
In the non-human primates (monkeys) the studies showed far superior results and it was comparable to many other published studies given out by other companies. This was done in government labs, including at ICMR-NIV because we can't do the live virus testing. Their results showed very strong data on this platform.
Is it true that with the DNA-based platform, the ease and speed of making the vaccine constructs is many times more and that is what makes it a potential game changer?
That has been our view and now the view of all scientists. If you want to use it for new constructs, this is one of the easiest platforms to do that. We have already completed our development for almost eight strains of Covid-19. We have started our animal studies to see if any of those strains can be neutralised. That is the beauty of this platform—it needs just six to eight weeks. Also, if mixing of multiple strains is the way to get the best immunity, so that none of the viruses escape, this will offer that capability, too.
We want to make sure that the next tech/platform upgrade is continued so that we have vaccines with significantly good efficacy. [The vaccines we had developed for tackling the original Wuhan strain of the novel coronavirus lacked the efficacy to tackle the Delta variant].
For how long will your vaccine provide protection?
Currently, we are following up with our patients for six months to one year. It is difficult to say but in vaccines two things work: An early antibody response or antibodies-based immunity and then t-cell response or the cell-based immunity.
We believe that there should be long term efficacy but because it has been only six months now for the follow up to our trials, we will wait to see for how long we can prevent severe to moderate cases. I think that kind of data for a longer period of time is not statistically available for any other vaccine developed either, because it is too early to say that. We are also now monitoring our volunteers for a year.
Six to nine months is what seems to be the case with the others vaccines. Is that why booster shots are recommended?
I can’t answer that. Of course, we are seeing that antibodies wane away because this is the nature of antibodies for Covid but whether the cellular responses stay well and for how long, that depends. Also, there are strains that mutate. So only real-time data over a period of a year can answer that question. Having said that, I want to reiterate that we are very confident for at least six months of protection but we have to see how the data progresses.
DNA-based vaccines have been referred to as the third-generation vaccines and the WHO called this platform as the radical approach which offers advantages over traditional vaccines.
The biggest benefit is the plug and play technology of making this vaccine adaptable to new variants of concern and that can be done rapidly and safely. The second aspect is that for a large global vaccination, this is an extremely thermostable vaccine and this is proven by open vial studies too. So, in a way this will also help in reducing the whole vaccine wastage which the WHO spoke about earlier pointing towards 40 per cent wastage on vaccination.
And then vaccine hesitancy will also be taken care of by this Plasmid DNA vaccine as this is a needle-free application. Also, it is easily scalable as it is a BSLR level 1 manufacturing requirement so one doesn't need high biosafety requirements to make this vaccine. And, I strongly believe that going forward if a vaccine must be developed for tackling multi-variants, this is the one as it has an edge over the others to be able to do that.
What is the science behind going needle-free?
If it is DNA, it has to go all the way into the cell membrane and nucleus. The best way to do it is intra-dermal. Intra-muscular was becoming harder because of the use of lipids, which could create challenges in administering the vaccine and also other reactions related to it. Globally, there are a lot of capabilities for delivering intra-dermally, but those are very expensive. We were able to find a platform which was approved by the FDA. It is a high-pressured injection, an almost non-intrusive dose given in the upper skin, which is pain free and will have minimal side effects.
Why are three doses needed?
Currently, there is a three-dose trial—day zero, day 28 and day 56. The recipient is fully vaccinated after day 56. We saw a longer immune response by the body after three doses, when we did animal studies. It is a challenge because the vaccine needs to be given on the upper skin and also because of the amount of vaccine that needs to be absorbed. So if we were to give a higher dose in a single shot, it will be difficult for the body to absorb it. But in order to get an ideal immune response, I have to give 6mg and that would be possible only over a period of three doses. We are also working on a two-dose system, but the dosage will be the same—that is 6mg, but it will be over 28 days, instead of 56 days.
What have been the challenges you met along the way in the run-up to developing the vaccine?
We have a team of 120 who are working on this vaccine and they faced a number of hurdles along the way. Beginning with the number of samples that needed to be tested in government or private labs—nobody has the bandwidth for it because Covid was raging at the time.
In the meantime, as we were working on the vaccine and did not know if we would be successful or not, we took the risk of creating a large-scale manufacturing facility or fermentation plant for recombinant proteins which were readied in ten months.
Then the next challenge was the trial for which we got the 28,000 volunteers across 70 sites especially in the periods of April to June when things were really difficult and the covid caseload was high and so to get people to come for the vaccination trial was challenging. These factors also delayed us to an extent.
Did you expect to launch the vaccine nearly two years after your company first thought of it?
No. In our minds, it was March of this year but then it took time to complete the recruitment of volunteers for the trials. We also had over a thousand adolescents participating in the trials and so the recruitment itself went on until June.
It would have been very easy if we were only giving the vaccine but to achieve a vaccine that is effective and safe, we conduct clinical trials during which half of the volunteers participating in the trial are randomly selected and given the experimental or in this case the DNA vaccine and the other half is given a placebo vaccine.
All the other conditions related to trial are kept the same for both groups and none of the individuals in the sample is told if they have received the real drug or the placebo. Maybe because of this, it wasn't easy to recruit children, because they did not know whether they were being given the vaccine or the placebo.
You say the safety, immunogenicity and efficacy of the vaccine are well established. How does it compare with vaccines already available in India?
I don’t think there is a head-to-head comparison, but if you look at globally published papers... we are comparable with many of the prominent platforms. [As far as the overall efficacy of existing vaccines,] we are currently at 67 per cent in terms of getting Covid-19. [With our vaccine], as of now, we do not have any moderate and severe cases reported. Moreover, nobody has controlled data on the Delta variant as of now. All the earlier data was of the Wuhan strain. Our trial was during the Delta period, so we can say for sure that our vaccine will take care of the Delta cases. Even serosurveys suggested that 99 per cent of all cases in India were Delta cases and when we did the subset of our positive cases, all of them were Delta cases. So our data is more current to the Delta variant.
Is the plasmid DNA vaccine better for children?
Our vaccine does not have any adjuvants or infectious vectors, so the side effect profile will be far better tolerated. That is why this platform was approved to be tested on adolescents and now in younger children.
What about extending the coverage to children below 12?
We have only started. Now the new trial has just received approval and soon we will begin trials for children aged 5 to 12.
Is there a need to repeat the doses annually or bi-annually?
We can't say as of now. We are early in this phase and have to study it. If necessary, we will come out with the booster doses, and it will be for the delta variant.
If you talk about protection in moderate to severe cases we are doing very well, but in the case of mild Covid our efficacy is around 67 per cent. There is no data as of now that can prove long immunity.
If a more virulent, transmissible and infectious variant is to appear in the coming months, will your vaccine hold good against it?
It is too hypothetical and hence difficult to answer.
How do you analyse the impact your vaccine might have on your stock price?
No idea.
Are you in talks to export the vaccines abroad?
We have a lot of requests for that but with our current capacities, we are only committed to India. As of now, we do not have the capacity to commit to exports as of now. We are also evaluating the prospects for partnering with other countries.
Do you expect the third wave to hit us anytime sooner?
It would be there but not severe.
What's next for your company from here on? Also tell us about your foray into developing monoclonal antibodies.
Our efforts in the vaccine space will continue not just for Covid-19 but our overall vaccine program which comprises of 11 vaccines approved. For Covid, beyond vaccines we are also into therapeutics that are providing solutions for when people do get infected.
One aspect of creating the cocktail of monoclonal antibodies is ongoing and we are in clinical phase now. (Monoclonal antibodies are laboratory-made proteins that mimic the immune system's ability to fight off harmful antigens such as viruses. In other words, it means developing antibodies by inserting them in the human body.)
We are working very aggressively on monoclonal therapy as the early administration of this therapy does prevent severity in patients who have comorbidities.
We will complete the trial in three months and the product will then have very strong antibodies which will stay in the human system for much longer than the current standard of care which will protect people when they get infected and the side effects will also be lower. Research on easier diagnostics including a one-minute RT-PCR tests and more comprehensive covid testing is ongoing.
Is your vaccine also for children under five?
Not as of now. Our current effort is only five years and above. If we do well and successfully in this age group, we will see if there is a need to go below that age group.
What's your immediate goal?
To see to it that our vaccine protects above 95 per cent from the current 66.6 per cent. That journey will continue for all of us.