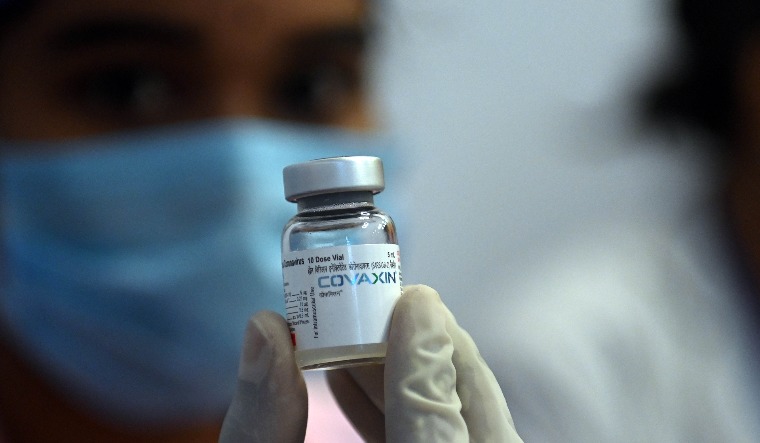
Indians who have received the indigenously developed COVID vaccine Covaxin may have to face more delays on plans to travel overseas. Media reports on Monday and Tuesday said that the WHO's emergency use authorisation for Covaxin is likely to be delayed by a few more weeks.
News 18 reported that the WHO had raised questions on Covaxin, which had been sent to Bharat Biotech, its manufacturer. News 18 quoted a source as saying, "It’s the usual process. Experts raise queries, which need to be answered by the company."
An EUA from WHO is a major step towards recognition of Covaxin by other nations. Covaxin is yet to be accepted by most countries.
Bharat Biotech had announced it had submitted all documents required by the WHO for emergency use listing (EUL) of Covaxin by July 9. The company had said in July that approval from WHO was not expected to be a "long drawn process".
also read
- India second highest in hepatitis B and C after China, says WHO report
- India accounts for second highest number of cases in hepatitis B & C after China: WHO
- WHO says Gaza has only 11 partially functioning hospitals out of 36
- Health effects of loneliness equal to smoking 15 cigarettes a day: WHO
- Time to cut down on artificial sweeteners? WHO finds Aspartame 'possibly carcinogenic'
Last week, Union Minister of State for Health Dr Bharati Pravin Pawar was quoted by ANI as saying "WHO's emergency use authorisation to Covaxin is expected soon”. V.K. Paul, chairman of the National Expert Group on Vaccine Administration, had also claimed WHO approval for Covaxin could come before the end of September.
News 18 reported as of Tuesday "over 9.28 crore doses of Covaxin have been administered in India".