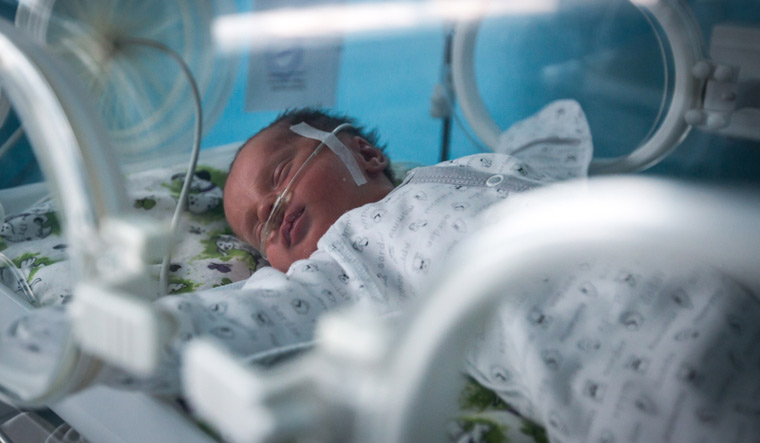
Nestle, a key manufacturer of breast milk substitutes for infants, is under the scanner for conducting clinical trails on 75 premature babies in five different hospitals.
Nestle got doctors in five private hospitals to observe the growth patterns among 75 premature babies in the bracket of 28-34 weeks of gestation during their hospitalisation and admission in the Neo-natal Intensive Care Units. The study, titled, 'Multi-centric Observational Study to Observe Growth in Preterm Babies,' is against the very law which protects and promotes breastfeeding in the country, namely, the 'Infant Milk Substitutes, Feeding Bottles, and Infant Foods (Regulation of Production, Supply and Distribution) Act.'
"This is a gross and blatant violation of the IMS Act and demands strict action by the authorities as an offence committed under the law is cognisable. BPNI was recently alerted to the fact that Nestle is brazenly flouting the Indian law which prohibits such manufacturers from sponsoring or funding medical research or financing any part of the healthcare system," said Dr Arun Gupta, central coordinator of the Breastfeeding Promotion Network of India (BPNI).
The collected data would have helped the company in gaining additional information to embark on the mission of manufacturing and marketing its own branded formula milk for preterm babies. However, even the desire to do this goes against the age-old practice of breastfeeding, which is considered to be vital for the baby's growth and healthy development.
The company is a manufacturer of infant milk substitutes and infant foods and all such products come with the disclaimer that they are not substitutes for mother's milk. This research was undertaken to establish and promote its product range for mainly preterm babies, says BPNI. The five hospitals include Cloud Nine hospital and Manipal Hospital in Bengaluru, Institute of Child Health in Kolkata, Sir Ganga Ram Hospital in New Delhi and the Calcutta Medical Research Institute in Kolkata.
While the union health ministry has demanded the country's apex health research agency that the research be stopped, it has turned out that it was given the green signal by the ethics committees of all the five participating hospitals. According to the release by BPNI, 'this is nothing new for the company, their aggressive promotion of breast milk substitutes is unending inspite of the law and World Health Assembly having adopted guidance to end inappropriate promotion.' The group has written to Dr Harshvardhan. union minister of health and family welfare saying that the study is 'not even approved by any independent ethics committee' and requesting the initiation of action against the company and the five hospitals.
According to Nestle India, the objective of the clinical study is to encourage science based research. "This study is an institution based study, all Institutional Ethics Committee approvals have been obtained from the participating sites. The letter from the Ministry of Health and Family Welfare (MoHFW) has requested Indian Council of Medical Research (ICMR) to examine the matter. Nestle India will provide all its support on this issue to ICMR and we are confident of our position," said the company spokesperson.