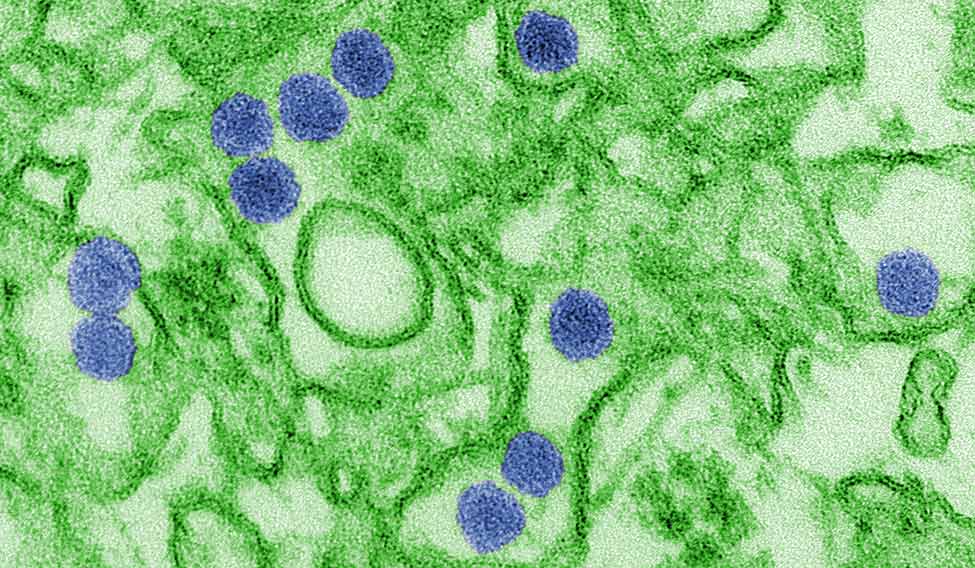
Rio de Janeiro
15 January 2016; 2.17 p.m.
A young woman named Maria was bitten by a mosquito (species unknown). The mosquito’s salivary glands slid an infusion of Zika Virus into Maria.
This is an account of the virus’s sojourn in Maria’s body:
2.18 p.m.
Zika Virus enters Maria’s skin. A water-slide of mosquito saliva swooshes it on the spongy connective tissue of the dermis. The skin’s guardian cells are everywhere. These dendritic cells, immature laggards that hang about, are equipped with sensors to detect intruders. That is okay with Zika Virus. It wants to be found.
The AXL receptor on the dendritic cell glows and Zika Virus is swept up in its welcoming arms.
There are other cells too that Zika Virus can visit—keratinocytes, fibroblasts. These are docile cells capable of what the dendritic cell is doing this very moment to Zika Virus—slurp, burp—phagocytosis.
That was quick!
2.30 p.m.
Zika Virus now is exactly where it wants to be—within the cell.
It has been conducted into the cytoplasm by endocytosis and has been allotted an acidic endosome. Not quite what’s due to a positive-sense RNA virus, but Zika is not complaining.
The cell’s housekeeping recognizes the error. Zika Virus, being so similar to the cell’s own RNA, deserves an upgrade.
So, Zika Virus is permitted into the ER.
Within the hour, Zika Virus has got its act together.
6 p.m.
Zika Virus has formed autophagosomes, and has begun Xeroxing itself.
8.30 p.m.
The Zika Virus Next Generation is piling up in autophagosomes at a frenetic pace.
17 January 2016
Zika Virus relaxes a little. It has done well. More than 109—a billion—sons and daughters wink back from the autophagosomes.
Time now to take stock.
First, other receptors on the host cell membrane could help AXL.
It is good to have more friends in the enemy camp: DC-Sign, Tyro, Tim-1 and Tam.
More receptors means more cells to infect, perhaps even more kinds of cells. But who wants trouble? Best stick to hospitable cells with plenty of AXL for easy entry.
Meanwhile, Zika Virus can’t overlook the changes occurring in its immediate environment. The host cell has woken up. It is organizing the home guard.
Even as Zika Virus’s rough little virions are being shooed and shushed into a semblance of viral decency, the host cell has set off loud alarums. These are signals set off by Toll Like Receptors to encourage secretion of the inflammatory chemicals, cytokines and chemokines. More notably, the host cell increases the production of interferons.
That is bad news for Zika Virus.
The inflammatory response set up by interferons obstructs Zika Virus’s plans.
Maria begins to feel ill. This induces her to rest. No longer compelled to squander energy on physical activity, her body diverts resources to the immune system.
Interferons whistle up every possible sort of cell that is capable of putting up a fight—macrophages, natural killer cells, lymphocytes. They all queue up.
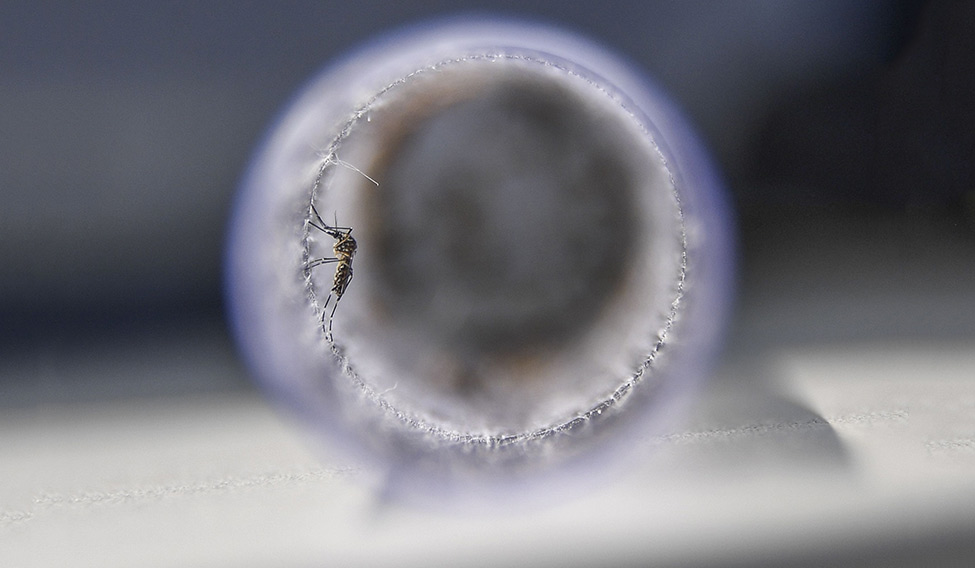 Sting in the tale: An Aedes aegypti mosquito infected with a bacteria that prevents it from spreading dengue, Zika and chikungunya, before its release at Ilha do Governador in Rio de Janeiro, Brazil, recently. Such mosquitoes will infect others in their species, reducing the population that transmits the diseases | AFP
Sting in the tale: An Aedes aegypti mosquito infected with a bacteria that prevents it from spreading dengue, Zika and chikungunya, before its release at Ilha do Governador in Rio de Janeiro, Brazil, recently. Such mosquitoes will infect others in their species, reducing the population that transmits the diseases | AFP
Even worse, the signals for apoptosis have been switched on earlier than Zika Virus would wish for. Its harum-scarum young still have to be put through finishing school in the Golgi Complex before they can be unleashed into the circulation. If the host cell caves in now, the juvenile virions will be no match for the killing squad lined up outside.
Zika Virus isn’t very certain if it can block interferons. Its cousins Japanese Encephalitis Virus (JEV) and Dengue Virus (DENV) surely can.
Zika Virus understands it has some pioneering to do. It will have to junk some of that ancestral reticence towards a human host. It has, after all, rearranged its amino acids into a recognizably new French Polynesian strain, of the Asian prototype. That should mean something.
What did those chaps in French Polynesia pull off exactly? There’s no time to check on that now—Zika Virus feels a seismic shudder through the autophagosomes. The host is having some kind of a crisis. Its long lazy siesta is over. Carrying Zika Virus’s billions within its ER, the host cell begins its journey to Central Intelligence.
18 January 2016
Zika Virus is on the move. The dendritic host cell has peeled itself off its cozy mattress, and stepped into the nearest lymphatic channel. Zika Virus is glad of the cruise. The skin was getting too hot for comfort.
Maria is running a fever. Replication has slowed down since the interferons perked up, but that isn’t really as worrying as the tumult within the host cell. The dendritic cell has woken up to its job, and is gathering intelligence on Zika Virus.
19 January 2016
A momentous day at Central Intelligence. This is the nearest lymph node.
The dendritic host cell has spent the past hour spilling the beans on Zika Virus and transforming T-lymphocytes in the lymph node. They are organizing for chemical warfare.
Time for Zika Virus to get out. The autophagosomes are so burdened with viral protein now that the UPR signalling pathway buckles and apoptosis is triggered.
As Zika Virus steps neatly out of the dying cell, apoptosis also releases the Next Gen Zika Virus zillions.
Freedom at last!
Eager young viruses jostle their way to the blood stream. They’re waylaid by all manner of killer cells, confused by conflicting chemical signals, and refused entry into many interesting places, but who gives a damn? They overwhelm every obstacle by sheer numbers as they charge through the circulation.
Now, if only Maria would get bitten by another mosquito, one part of Zika Virus’s agenda would be satisfied—enough young viruses could jump aboard the mosquito. After a wild vacation—the Extrinsic Incubation Period—they would then enter a second host.
Zika Virus is beginning to think this might be the end of the line within Maria. A few other replicating units could perhaps be managed, but none of the tissue types seem particularly interesting.
Unless—wait!
This is beyond Zika Virus’s wildest expectations. None of those old guys in French Polynesia even mentioned this. It is not part of viral memory, and yet, Zika Virus knows exactly what to do. Charged for a new adventure, Zika Virus reviews its strategies.
Maria is no ordinary human.
Within Maria there is a hot little nubbin of virus heaven.
Maria is pregnant.
Just ten weeks old, Maria’s baby will soon qualify for the label ‘foetus’. It has zoomed up from a clump of cells into something recognizably human. It is, however, still shielded from Zika Virus by the placenta.
Zika Virus doesn’t, yet, recognize it as a new host.
Ah, the placenta.
That is worthy of Zika Virus’s attention.
True, ancestral wisdom falls short here. Zika Virus has never been known to cross the placenta. But every virus knows, the entire point of survival is to boldly go where no virus has gone before …
Zika Virus quickly scans its arsenal.
After zipping through the circulation, Zika Virus is wiser about the host. There are places in Maria’s body where the usual rules don’t apply. Here, the immune system, both innate and adaptive, may look the other way, and actually benefit Maria.
It is called Immune Privilege.
Zika Virus is aware of three such places: the brain, the genital tract, and the placenta.
Zika Virus picks the brain first. That’s the traditional choice, neurotropism runs in the family. JEV, West Nile Virus (WNV) are brain veterans, of course—but even low-lifes like DENV and CHIKV make a bid for encephalitis now and then.
Besides, family lore hints at a shadowy reputation for neurotropism in Zika Virus itself, although that was in a different country and the host was a mouse.
Certainly worth a try.
When Zika Virus tries to sidle past the Blood-Brain Barrier, it gets bounced out.
The genital tract is more iffy—Zika Virus is not certain of any gains therein.

20 February 2016
So placenta it is!
The news is that the placenta also has a Barrier.
Zika Virus does a recce.
The Placental Barrier is just getting its final coat of paint when Zika Virus arrives. The host’s optimism is pathetic. The vaunted Barrier is nothing but a fringe of foetal tissue waving about in a puddle of Maria’s blood. This should be easy.
Not entirely. It turns out to be more complicated than Zika Virus imagines.
Each wave of the fringe is a villus. Its wall is made up of syncytiotrophoblast* cells. The foetus converses with Maria through these cells by constantly exchanging nutrients.
And here’s something curious—Maria’s immune cells too can’t seem to get past the syncytiotrophoblast. So the foetus doesn’t have any of her educated T-cells, armed to the teeth to kill viruses.
Now, if Zika Virus could only scale the wall …
The syncytiotrophoblast is a tough wall to breach, but Zika Virus notes a few weak spots. The wall is renewed by cells from below—from the next layer, the cytotrophoblast. Besides the free floating villi, there are anchoring villi also—here the cytotrophoblast burrows into the wall of Maria’s uterus.
Zika Virus now gets some alarming news about the core of the villus: lurking about the central foetal blood vessels are Hoffbauer cells. These are the macrophages of the placenta, capable of detecting and presenting antigens, on the prowl for intruders, and sweeping the placenta with their sensors.
This isn’t Mission Impossible. It has been done before. Other viruses have breached the placenta with ease, and it is worthwhile taking a quick look at their strategies.
Cytomegalovirus gets past the syncytiotrophoblast and sets up replication in the core cells.
A little earlier—say a fortnight or so—when the trophoblast cells are still immature, brutish fellows like Adenovirus and Herpes Simplex simply blast their way across. At the other extreme, there is transcytosis.* Get in at one end of the cell, stroll sedately across the cytoplasm, then exit without a fuss. Hepatitis B does that so well.
Zika Virus decides on its target. It will zoom in on the cell that orchestrates the entire immune response in the placenta—the Hoffbauer cell.
Zika Virus scans the villi and finds it isn’t difficult to slip past the syncytiotrophoblast and set up shop in the cytotrophoblast cells. Here it begins replicating—from here to the Hoffbauer cell it is just hop skip and jump. Do the Hoffbauers have Zika Virus friendly receptors?
Yes! Receptors on the Hoffbauers engage with Zika Virus, and very soon it is inside the Hoffbauer ER, and Xeroxing away! Hurray! It has discovered Immune Privilege. It works like this:
The Hoffbauer cell, having internalized Zika Virus, should set off a strong interferon response, summon all immune cells, encourage a flood of chemokines, cytokines, and the entire gamut of inflammatory chemicals.
This doesn’t happen.
That is Immune Privilege at work, which commands the placenta to be kind to the non-self. So when alarms flash and bells ring, the Hoffbauer cell takes a chill pill. It doesn’t break out in a sweat of inflammatory chemicals. It doesn’t get suicidal and begin apoptosis. It maintains a detached calm and allows Zika Virus to quickly form autophagosomes in the ER and start replicating.
Zika Virus hunkers down and works overtime till its Next Gen is ready for exit. The Hoffbauer cell is now pushed into apoptosis and the sons and daughters of Zika Virus burst out, envelope the foetal blood vessels at the centre of the villus, and BAM!
Zika Virus has slammed the foetus.
As it enters foetal circulation, Zika Virus can scarcely contain its excitement. Its target tissue is just a pirouette away—the nest of developing, differentiating, neural tissue. The foetal brain.
At ten weeks, the foetal brain already has had a momentous past. In the fourth week, the Neural Tube changed shape and developed three pouches. Lining the cavity are Neural Progenitor or Precursor cells. These stem cells have a program to form neurons that can be sent out to fashion different parts of the brain and spinal cord.
Zika Virus has no ancestral memory of the human brain. Having knocked about in Maria now for almost a week, it is evident how huge her brain is. Billions, maybe trillions of cells. How is the tiny group of Progenitor cells in the 9 mm brain factory of the foetus ever going to match up?
Zika Virus decides this is worth watching.
By now, the Neural Progenitors have been replicating fast enough to earn Zika Virus’s respect. By the sixth week they had produced enough cells to delineate the future shape of the brain. Within the brain’s cavity, the ventricles, lies a vigilante structure that Zika Virus notices with some trepidation: it is the choroid plexus. When developed, the choroid plexus will form a barrier between the circulating blood and the cerebrospinal fluid that laves and nourishes the foetal brain.
Zika Virus is impressed by how efficiently Neural Progenitors divide. They do so asymmetrically by replicating parent cells and Next Gen neurons and glia.
Zika Virus also notices that a special group of Neural Progenitors, radial glia, send out cell extensions that act as scaffolding for migrating neurons. This process will continue till the eighteenth week, after which neuronal migration ends, and radial glia will become transformed into astrocytes.
Zika Virus is still working out the right approach. Being in the swim of foetal circulation isn’t nearly enough. There are other walls ahead.
The foetal brain has its barriers too.
The Blood-Brain Barrier and the Blood-CSF Barrier. Zika Virus can choose to get past either of these—or both.
Between the inside of brain capillaries and the surface of the brain is the Blood-Brain Barrier. It is made up of the endothelial cells of the capillary wall, astrocytes, and pericytes.
Astrocytes seal off the capillary wall by clamping down their ‘end-feet’ over cell junctions to make them ‘tight.’
But they can’t be easily dismissed as rude mechanicals. They’re smart cells that ‘sense’ intrusions of all sorts. Pericytes crouch over the capillary wall like benign toads on a log, ready to jump at the first tremor. They nurture and protect the developing brain, and seem more parental than supervisory in their controls. They regulate blood flow through the capillaries by relaxing and contracting according to demand. When more blood is needed, as the oxygen demand increases, pericytes relax. In addition, they have locator functions. Pericytes in the middle of a capillary maintain the Blood-Brain Barrier. Those at the arterial end of the capillary regulate blood flow. Pericytes at the venous end monitor the entry of immune cells into the brain.
Zika Virus discovers more interesting facts. The brain has no lymphatics, so if Zika Virus were to set up shop within a neuron, there is little chance of inflammatory cells sweating out interferons.
There is also the Blood-CSF Barrier located in the choroid plexus within the ventricles: this controls the passage of molecules into the fluid bathing the brain.
And then there are microglia—scavenger cells that safeguard the foetal brain from infection. Like dendritic cells in the skin, these microglia are shape-shifters too. They have small fixed bodies and long mobile processes which constantly sweep the brain to sense the slightest change in its milieu. At first suspicion, they change into fast-moving amoeboid cells and hurry to the site of injury or intrusion. Here they may grab the intruder and engulf it and start off the tedious chemical warfare Zika Virus knows so well.
Zika Virus notes that because of the rapid multiplication of Neural Precursors, apoptosis is common to regulate the number and distribution of neurons. Amoeboid microglia swarm areas of apoptosis to clear away debris.
Now that it knows the enemy, Zika Virus reviews its strategy.
There is a lot of family lore to draw from.
Many flaviviruses are neurotropic. Zika Virus takes heart from the fact that flavivirus neurotropism evolves rapidly. DENV, for instance, doesn’t usually go for the brain—but it can, on occasion, when it behaves like JEV and WNV, causing paralysis or Parkinsonian symptoms. What brings about this change? A mutation in the viral capsule.
JEV targets immature nervous tissue—hitting out at the hippocampus, a part of the brain where Neuronal Precursors stay on even after birth.
Zika Virus ponders questions that have no answers as yet:
What makes a flavivirus neuro-invasive?
Why is it specially neuro-invasive when it emerges?
Why does it target immature or developing neural tissue?
Memory answers with a ghostly chuckle:
Molecular mimicry.
What the—
Right! It all comes flooding back.
Molecular mimicry is where infectious agents activate the body’s immune system into the self-destructive process of auto-immune disease. If a molecule present on the pathogen is similar to one present on a body cell, the immune system can confuse the two.
How?
It is all about sialic acid. Common stuff, it drenches nervous tissue and goes about making synapses, connections between cells. Foetal brain should be bursting with sialic acid compounds—gangliosides, polysialic acid.
Viruses have this stuff too. As do bacteria.
So Maria’s immune system recognizes these compounds as dangerous—and makes antibodies against them.
And here’s the trick of it.
Maria has made antibodies against Zika Virus already. After a few weeks, these antibodies will cross the placenta. And the Blood-Brain Barrier. What then?
When they encounter the ganglioside-rich developing neurons, it will be a massacre of the innocents.
And Zika Virus doesn’t need to do a thing.
Except sail in through the weakened Blood-Brain Barrier, get straight to the Neural Precursors and become a dedicated killing machine.
Yes, that might be a neat plan.
But here’s something neater still.
In Maria’s bloodstream, Zika Virus recognizes the footprint of a not-so-distant cousin—Dengue antibodies! DENV1 has had a long vacation in Maria, and left her immune to just that one branch of the family. Maria has antibodies against DENV1.
Should she play host to DENV 2 3 or 4, all of which hover in passing mosquitoes, Maria could be in bad trouble.
These very antibodies she has made against DENV1 could actually help the new guys. They could opsonize them.
Zika Virus hasn’t considered this so far.
That’s a tasty word, opsonin. It means ‘to make something palatable’. The DENV antibody can grab the new guys and literally make them tastier. Phagocytes can’t wait to gobble them, and in go the DENV newbies, right into the ER to set up autophagosomes and start Xeroxing. So an earlier DENV infection makes replication faster. More host cells participate, and who knows what barriers will be breached?
This trick even has a special name; Antibody Dependent Enhancement (ADE).
And if DENV 2, 3, and 4 can get the ADE advantage, why shouldn’t Zika Virus?
They’re kissing cousins after all. What works for the DENV might just work for Zika Virus too.
Zika Virus pushes for an introduction.
And—guess what?
It works!
Suddenly there is a lot more of Zika Virus everywhere.
Zika Virus, organizing its placental factory, is still biding time …
 AFP
AFP
28 February 2016
The placenta has changed. Zika Virus’s autophagosomes encourage apoptosis. There is more cell death than is expected. With all this interferon triggering, you might have expected Zika Virus to hold back.
No siree!
Zika Virus has another trick up its sleeve—one that its ancestors had no opportunity to try out.
(Maria, it is worth remembering, is a new kind of host. She is human.)
Zika Virus’s Non Structural Protein 5 knocks out Interferon-Type 1 very definitely, and Type 111 very possibly. It does this by binding and degrading STAT-2 which up-regulates hundreds of interferon genes. With STAT-2 knocked out, all these genes blink out, and the interferon response is dampened. Considering that interferon signalling is the strongest anti-viral response, this is a very very big deal indeed.
The placenta is a terribly busy place. Like a Bombay railway station during rush hour, the chaos is indescribable, and yet, everything, miraculously, is on schedule. Like a thaumaturgist pickpocket Zika Virus effortlessly homes in on placental cells that seem vulnerable. As the AXL receptor is expressed strongly throughout the first trimester, Zika Virus is welcomed by many trusting cells.
The immediate result?
Massive cell death results in scars and constricts the blood vessels.
Desertification of the placenta begins. Those warm pools of maternal blood are choked into tight little gutters that villi can barely dip their toes into.
The foetus is being slowly starved. Its growth begins to lag.
Ides of March 2016
Zika Virus enters the foetal circulation.
It now approaches the brain armed with several stratagems. And, all of them work.
In the Blood-Brain Barrier, the astrocytes are particularly rich in AXL receptors. This one’s a natural. Zika Virus gets pulled into these cells and sets off the trigger for apoptosis— and disrupts the Blood-Brain Barrier to enter the brain.
That is one route of entry.
Zika Virus can also hitch a ride by transcytosis through an inflammatory cell like a monocyte once the Blood-Brain Barrier becomes ‘leaky’.
Whatever its mode of entry, it is there now, in flavivirus heaven. It has finally made it to the motherlode of pleasure, immature nervous tissue, still in the process of differentiation, the Neuronal Progenitor Cells.
These cells are located in the Ventricular Zone, and Zika Virus quickly finds its way there. These cells are in several stages of development—there are parent cells, Neuronal Progenitors, and Radial Glia; there are differentiated neurons in the process of migration; and there are microglia, the scavenger cells of the brain.
Which of these will Zika Virus choose?
All of them.
Neuronal Precursors and Radial Glial cells are easily subverted. Zika Virus quickly enters them by endocytosis and forms autophagosomes and starts to replicate endlessly.
The effects on the brain are lethal.
In the coming weeks … through the second trimester of Maria’s pregnancy, this is what Zika Virus achieves:
* The brain’s grey matter, the cerebral cortex, is thin and deficient. After 20 weeks its surface should begin to bunch into folds and grooves to accommodate a wealth of neurons. This does not happen in Maria’s foetus where the cortex remains flimsy and smooth. Consequently, the skull bones lag in growth. Maria’s foetus now has a small smooth brain in a very small skull.
* The eye is an extension of the brain. Zika Virus discovers that it can infect the eye even in the absence of AXL receptors.
* Zika Virus finds it easy to tinker with genes that signal specific functions in Neural Precursors. It down-regulates genes that deal with DNA replication and repair. It up-regulates genes that manage the UPR response and apoptosis.
* Meanwhile, the injuries to the placenta continue, and Maria’s foetus has severe growth retardation as well.
In the fifth month of her pregnancy, Maria’s foetus dies.
When the dead foetus is delivered, Zika Virus is found in the amniotic fluid and in the placenta.
The foetus has a small skull.
The brain within is smooth, with very few folds. The cortex is thin.
Many parts of the brain are misshapen and malformed.
Wide swathes of cell death are noted on microscopy.
Astrocytes are bursting with autophagosomes which are full of replicating Zika Virus.
The eyes are malformed.
The arms show contractures—arthrogryposis.
Zika Virus has reached a dead-end in Maria’s foetus. Crossing the placenta and killing the foetus was a bad idea, it was an evolutionary failure.
Would infecting the foetus a little later into pregnancy, when the placenta allows the passage of antibodies, have been any smarter?
In that case, the baby would have been born without microcephaly, but the brain would still have had plenty of place for Zika Virus to set itself up. And when the baby was born, Zika Virus could induce enough apoptosis to achieve viremia and swim free in the baby’s blood, waiting for a mosquito to bite ...
Extracted with permission from Speaking Tiger.

The Secret Life of Zika Virus
By Kalpish Ratna
Published by Speaking Tiger
Pages 271
Price Rs 299