Given the dread, discomfort and pain involved in the use of the injectable insulin, an inhalable form is indeed a boon for diabetes patients. With the first inhalable insulin called Exubera marketed by Pfizer in 2006, one would have expected a new insulin delivery system to bypass the constant need for a needle and syringe. No more than a year into its release, Pfizer decided to stop the production and marketing of Exubera. Other companies involved in the research and development of their own form of inhalable insulin, too, stopped their research following the withdrawal of Exubera.
In 2014, FDA approved an inhalable insulin developed by Mannkind, its marketing partner for the same being Sanofi. The drug hit the US markets in 2015 but is claimed to have suffered heavy loss, even before it could hit the world markets. Due to this, Sanofi pulled out of its agreement with Mannkind early this year, citing the same problems that Pfizer faced with Exubera which was lack of popularity and profit. Mannkind has, however, assured that the drug will continue to be available in the market. According to some reports, the company is planning to relaunch the drug in July.
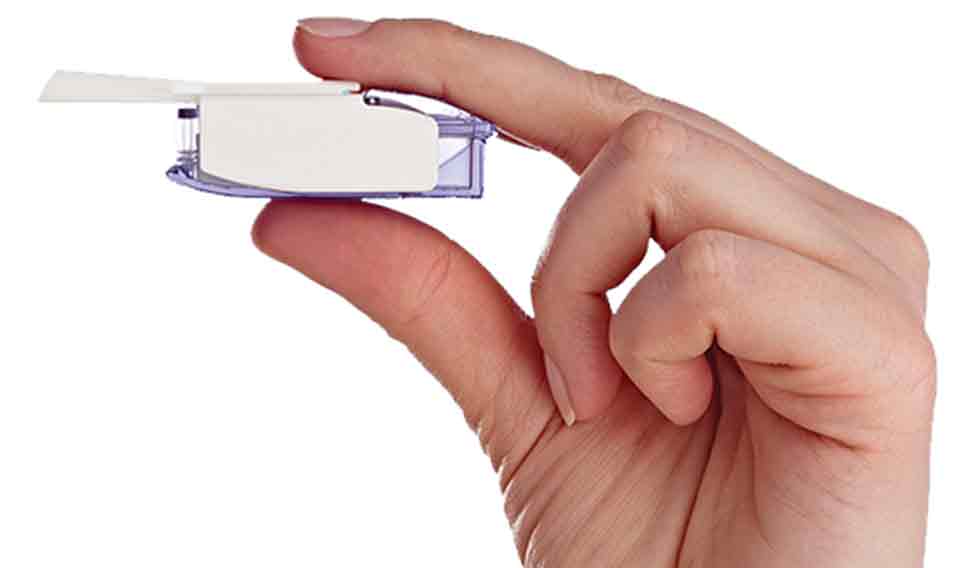
Clinically, Afrezza has shown promise with no drawbacks as compared to injectable insulin. It can be used for immediate reduction of blood glucose such as that required by diabetics after meals. It is not an alternative for long-acting insulin and patients who are smokers or are having lung disorders such as COPD or asthma should avoid using Afrezza. Compared to rapid-acting insulin, it is slightly inferior in its glucose-lowering ability but has a lower incidence of hypoglycaemic episodes. Also, its kinetics is fastest when compared with other forms of insulin. The device used to deliver the powder is the size of a whistle. The patient can comfortably use it in public without the physical and mental discomfort of using injectable insulin. The device is used with a cartridge that contains the insulin powder which is available in 4, 8 and 12 units. The delivery system is effective in reducing the number of injections required by most patients, if not in eliminating the need for the same altogether.
Fearing that the production of Afrezza might come to an end, diabetics in the US had demonstrated their disappointment on social media and on online forums while also expressing their satisfaction with the product. “So disheartened to see that @sanofi dropped #Afrezza. This drug has made managing my daily life 10x easier and I can't go back. #t1diabetes”, claimed one user on twitter.
Finding an alternative for injectable insulin has probably crossed every drug developer's mind at some point, with the clinical success of Afrezza, which is a fast-acting insulin approved for both type 1 and type 2 diabetes mellitus in adults. It would be a shame if this drug does not make it to the global market and gets withdrawn just like Exubera or, like one doctor put it, it would just be good science and nothing more.
Raghuvir Keni is doctor of pharmacy student at Manipal College of Pharmaceutical Sciences, Manipal, Karnataka.