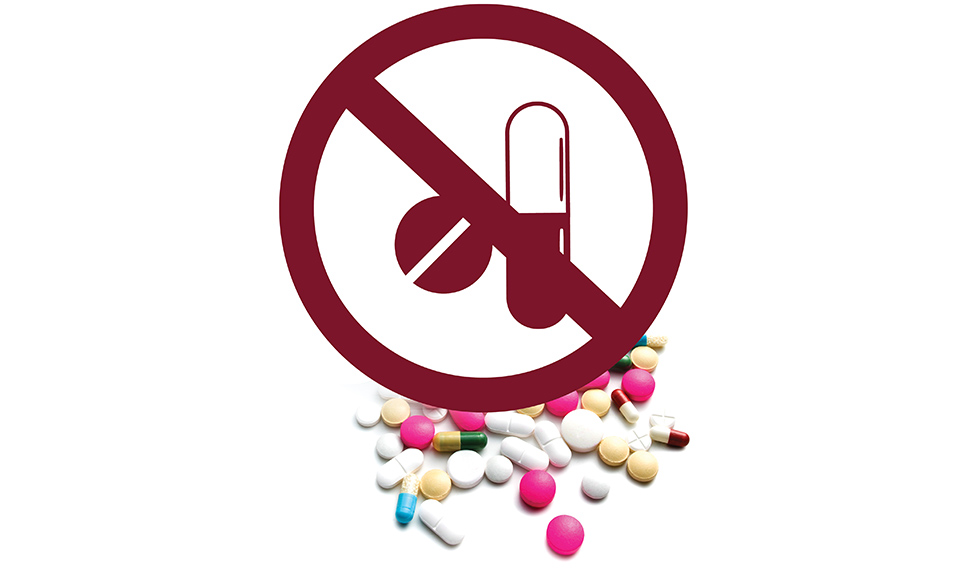
On March 10, 2016, the Union health ministry came out with a notification banning 344 fixed-dose combination (FDC) drugs. The pharmaceutical sector went into a tizzy, patients panicked, chemists tried to compound the matter with their own combinations, but confusion reigned. The notification was a buzzkill for junkies like Ajmal Sheikh, too. In 2013, the 20-year-old Mumbai resident had been prescribed Corex cough syrup for his persistent cough and cold. But soon, he started abusing Corex—a combination of chlorpheniramine maleate and codeine phosphate developed by Pfizer—downing two-three bottles in a day with friends. Doctors say codeine, which is an opiate drug, is highly addictive and is very commonly misused by people. The cough syrup was one of the banned FDC drugs.
Before the ban, says Sheikh, he would buy Corex cough syrup without a prescription from his hometown in Uttar Pradesh. In Mumbai, it was difficult to get it without prescription, but not impossible. “I discovered I could buy it for twice the rate in the black market,” he says. After the ban, his codeine addiction only became more expensive. “A 100ml bottle of Corex cough syrup costs around 190 and I would buy it for 1200 from my dealer, who sells prescription drugs right behind a police station next to my house. After the ban, the price shot up to 1300 for a bottle,” he says.
While Sheikh claims that he was able to buy the cough syrup illegally for a few months following the ban, Pfizer had discontinued the manufacture and sale of the cough syrup, which had been around in the market for nearly two decades, in March 2016 itself. Sheikh, who was forced to drop out of junior college owing to his codeine dependency, underwent a de-addiction treatment in December 2015, but had a relapse a few days after he was discharged from the hospital. Following the ban, he started working at his family’s grocery store and continued guzzling bottles of the cough syrup over the next few months. In November 2016, he was admitted to the hospital for the second time. “My mother finally put her foot down. I was admitted to hospital for two weeks. I feel like I can finally kick my habit. I want to stay clean and I also plan on completing my education for a better life,” he says.
 Dr R.K. Singal, chairman, internal medicine, BLK Hospital, Delhi
Dr R.K. Singal, chairman, internal medicine, BLK Hospital, Delhi
Drug abuse, perhaps, was one of the reasons that prompted the government to enforce the ban on FDC drugs. Corex apart, the ban affected the sales of other popular brands like Procter and Gamble (P&G)’s Vicks Action 500 Extra, Reckitt’s D’Cold, Piramal Healthcare’s Saridon, Abbott’s Phensedyl cough syrup and Glenmark’s Mucaryl cough syrup. The government's decision came after an expert committee, headed by Professor Chandrakant Kokate, vice chancellor of KLE University, Belgaum, found several FDC drugs were irrational or unsafe. The committee was set up on September 16, 2014. The final report recommended banning 1,083 FDCs after examining 6,214 FDCs, stating that they were irrational combinations. The report further recommended a study on other FDC drugs that were found to be rational owing to lack of adequate data on their safety and efficacy.
While this report was submitted to the Union government on February 10, 2016, the health ministry passed a notification banning the sale and manufacturing of 344 FDC drugs a month later. In the ban notification, the government stated that these drug combinations were likely to involve risk to human beings whereas safer alternatives were available.
On March 14, 2016, the Delhi High Court gave interim relief to pharma companies by staying the ban till March 28. The court said that other than stating that the use of the drug is likely to involve risk to human beings, the notification did not disclose any grave urgency. In April, the Karnataka High Court also granted interim relief to pharma companies based in the state.
Apart from pharma companies, the Delhi High Court's order was music to the ears of anxious chemists and druggists. However, on March 19, the Kerala High Court informed members of the All Kerala Chemists and Druggists Association (AKCDA) that they would have to return the banned drugs to their distributors within two weeks.
“We stopped purchasing the 344 banned FDCs and returned the existing stock over the next seven days. However, after the Kerala High Court order, there was no inspection from the state drug department. Pharma companies started sending us circulars that they got interim relief from the Delhi High Court and, in April, we continued selling these drugs,” says Thomas Raju, AKCDA general secretary. The stock of banned drugs that was returned was approximately 11 crore, he adds.
On March 22, the Madras High Court also refused to stay the ban notification. But the banned drugs were still available in several medical shops owing to delays by the Tamil Nadu State Drugs Control Department in implementing the ban. “In March, we returned our existing stock of the 344 banned FDCs to the distributors. We estimate that a stock of around 6,000 drugs worth Rs 100 crore was returned,” says S. Ramachandran, president, Tamil Nadu Chemists and Druggists Association. “However, following that, the drug manufacturers and distributors quoted the stay orders by the Delhi and Karnataka High Courts and started selling these drugs again.” While the Tamil Nadu State Drugs Control Department issued a circular to all drug inspectors to freeze the sale of banned drugs in the second week of November, the Delhi High Court revoked the ban on December 1. “After that, the petition was pulled out from the Madras High Court and the banned drugs continue to be sold in Tamil Nadu,” says Ramachandran.
The ban notification was set aside after Justice Rajiv Sahai Endlaw allowed 454 petitions to be filed by several major pharma companies including Cipla, Glenmark, P&G, Pfizer and Dr Reddy’s, challenging the government’s ban notification.
 Murlidhardas Subramanian, a diabetes patient | Janak Bhat
Murlidhardas Subramanian, a diabetes patient | Janak Bhat
The court set aside the ban on the grounds that the government had failed to consult the Drugs Technical Advisory Board and the Drugs Consultative Committee, set up under the Drugs and Cosmetics Act, 1940. The bench noted that the government had not properly implemented Section 26A [power to prohibit manufacture of drugs and cosmetics in public interest] of the act. Also, the court observed that a drug can only be banned following a three-month notice to the licence holder of that drug.
Soon after the ban, chemists and druggists associations went into panic mode as they weren’t given 90 days time to return the banned drugs. “Under the Drugs and Cosmetics Act, following the ban notification, we should have had 90 days to return the stock of banned drugs,” says Hukumraj Mehta, general secretary of the Maharashtra State Chemists & Druggists Association. “Also, one batch of drugs that we buy from a distributor is enough to sell for six months. We were not given any dates or batch number when we were told to return the banned drugs. There was a lot of confusion and the Food and Drug Administration [FDA] officials themselves didn’t know which drug combinations were banned.” But once pharma companies got interim relief from the Delhi High Court, he says, they started selling the banned FDCs again.
While the medical fraternity strongly supported the ban, many doctors were deeply disappointed after it was quashed by the Delhi High Court. “Many of the banned drugs were back in the market thanks to the stay order by the court,” says Dr R.K. Singal, chairman, internal medicine, BLK Hospital, Delhi. “As far as the public was concerned, there was no change following the ban and nor was there any hullabaloo among patients. Chemists and druggists didn’t want to lose money, so many of them first exhausted their stock instead of returning it. Entero-vioform, an antidiarrhoeal drug, was withdrawn from the market nearly two decades ago since it is unsafe. However, it is still freely available in Ghaziabad and smaller towns and villages.” The government, he says, did not complete its procedures with due diligence as it relied mainly on the Kokate committee report for passing the ban. Also, they didn’t have any data nor did they conduct any study to prove that the drug combinations were irrational.
Agrees Dr Urmila Thatte, head of clinical pharmacology at KEM Hospital, Mumbai: “While not all FDC drugs are bad, the problem in India is that there are no patents on drugs, so drug manufacturers have the liberty to make their own FDCs. No one knows how many FDCs are currently available in the market. Not even the Drug Controller General of India.”
Following the ban, other than a few popular over the counter medicines, several antidiabetic combination drugs were also unavailable in pharmacies for around a month. Murlidhardas Subramanian, 57, who has type 2 diabetes, was on Glizid MV by Panacea Biotec Ltd for six months before the ban. When Glizid MV—a combination of voglibose, metformin and gliclazide—was banned, he says he first approached his pharmacist instead of his doctors after doing some research online. “I knew I was taking a three combination drug and after it was banned, I ran a Google search and got the names of the three drugs. I then approached a local pharmacist and gave him the details. He then gave me a two-drug combination medicine and a single dose one,” says Subramanian, who runs a marketing firm in Mumbai. “I would like to know why these 344 combination drugs got banned and how they are unsafe for patients since I was using one of them. Despite following the news on this issue, it is still unclear how these FDCs can affect my health.”
Subramanian is not the only patient who chose to approach a pharmacist to find an alternative medicine following the ban. Doctors say only a handful of patients approached them for a new prescription. “Following the ban, we made attempts to call patients on three-drug combinations and change their prescription,” says Dr Sruti Chandrasekaran, endocrinologist, Gleneagles Global Hospital, Chennai. “However, the problem is that many patients on three-drug combinations don’t come back for a follow-up for months on end. It is difficult for us to keep track of how many patients are on a three-drug combination.”
Chandrasekaran says she has seen several cases where patients’ medicines were changed by pharmacists following the drug ban without first consulting a doctor. “I had a 53-year-old diabetic who was on a three-drug combination for almost a year. After the ban, the pharmacist recommended that she could take a two-drug combination and the third one separately. After one year of refilling her medicines without a doctor’s supervision, she finally approached me to verify if the medicines given by the pharmacist were right for her or not,” she says.
 S.V. Veerramani, president, Indian Drug Manufacturers Association
S.V. Veerramani, president, Indian Drug Manufacturers Association
This is a worrying trend, say doctors. “Many diabetic patients continue taking three-drug combinations twice or thrice a day, which causes diarrhoea. To get rid of that side effect, they have to be taken off that FDC completely. I was trained in the US where combination drugs are rarely prescribed. While using single dose medicines, I know which drug is causing a side-effect, which makes it easier to adjust the doses. For example, pioglitazone, a popular anti-diabetic drug, is known to cause weight gain,” says Chandrasekaran.
While doctors complained that the drugs ban had no impact whatsoever, pharma companies were forced to make some changes to their popular brands to recover the losses they suffered during the first two months of the ban. For instance, Pfizer announced that it would discontinue the manufacturing of Corex cough syrup, which had sales of 1244.48 crore from 2015-16. The cough syrup had been off the shelves of pharmacies since the ban was passed. In a statement, the company said it would be launching a series of products as extensions under the Corex brand name.
“We are fully supportive of any process that aims at weeding out medicines that are irrational or not duly approved by the central and state regulators,” a Pfizer India’s spokesperson tells THE WEEK. “It is important that such a process of identification allows all stakeholders to participate. Pfizer India did at that stage receive an interim injunction by the Honorable Delhi High Court that allowed us to continue the manufacture and marketing of Corex. Later, of course, the court has also made a definitive ruling in the matter.”

Pfizer India also undertook a comprehensive review of their respiratory offerings to be able to better cover a broader range of indications through an expanded product portfolio. “As a result of this, we decided to discontinue the manufacture of certain SKUs [stock keeping units], including the erstwhile Corex cough syrup,” says the spokesperson. “At the same time, we have decided to launch a series of line extensions of the Corex brand that will address specific sub-therapeutic areas under the broad respiratory segment. We will soon start bringing these products to market. Each product will have all due approvals from the central and state regulatory authorities.”
Following the ban, certain medicines that contained the banned FDCs were reintroduced into the market after changes were made to the ingredients. For example, P&G’s Vicks Action 500 Extra used to contain caffeine, paracetamol and phenylpropanolamine (PPA), which was one of the banned drug combinations. Following the ban, it was reintroduced in the market after PPA was removed as one of the active ingredients, say pharmacists. P&G is yet to reply to the email sent out by THE WEEK. Pharma companies like Dr Reddy’s, Piramal Healthcare, Cipla, Sun Pharma and Wockhardt refused to comment on the issue.
Meanwhile, Abbott started promoting variants of cough syrup like Phensedyl LR which is non-codeine and doesn’t contain any of the banned drug combinations. In an email reply, Abbott’s spokesperson says, “Abbott welcomes the Honorable Delhi High Court's order, which has allowed our writ petitions. The judgment provides thousands of patients continued access to Abbott’s doctor-prescribed products, which have the necessary regulatory approvals for manufacture, distribution and sale in India.”
It is estimated that the pharma industry incurred losses worth around Rs 15,000 crore in a span of just five months following the ban notification, says S.V. Veerramani, president, Indian Drug Manufacturers Association. “Once the ban was passed, drug manufacturers decided to move away from the banned combinations,” he says. “Though it was done in a hasty way, they opted for drug combinations approved by the government so they have now recovered from the losses they suffered during the first five months of the ban.”
Doctors, however, are concerned that the banned drugs will find a way back into the market as the ban has been revoked. “Nimesulide, a fever and pain drug, was banned in India a few years ago but it is still available and patients continue to use it. Patients will continue using a banned drug if it is available as long as they don’t suffer from any noticeable side effects,” says Dr Om Shrivastav, head of infectious diseases department, Jaslok hospital, Mumbai. “The government and the FDA should come up with a process where defaulters are penalised.”
For now, the matter is back in court. Last month, the Union government approached the Supreme Court challenging the Delhi High Court order. It had earlier sought transfer of petitions pending before the various High Courts to the apex court. Despite several attempts to contact K.L. Sharma, joint secretary, health ministry, he remained unavailable.